Beneficial effects of the multi-strain probiotic preparation BaciMix on antibiotic-associated diarrhea in rats
Abstract
Dysbiosis is a common status of intestinal microbiota in modern society and is associated with many diseases. Diarrhea is a kind of dysbiosis and is frequently caused by imbalanced gut microbiota due to misuse of antibiotics. A lot of evidence has shown that probiotics can exhibit potential to alleviate the effects of antibiotic-associated diarrhea (AAD). In this study, Lincomycin was utilized to induce AAD in rats and then the effects of the multi-strain probiotic preparation BaciMix on this model were evaluated. The rat groups, including healthy control rats, AAD-induced rats, AAD rats with no treatment (self-healing rats), and AAD rats treated by BaciMix preparation, were analyzed regarding general health status, some immune indices, and intestinal microbiota changes. The results disclosed that the BaciMix preparation remarkably reduced the effects of the antibiotic regarding the diarrhea score and cecum thickness in the rats treated by BaciMix preparation. Additionally, the BaciMix preparation reduced pre-inflammatory cytokines, including TNF-a, and IL-6, while increasing the IgA in sera and intestinal mucosae. Moreover, the BaciMix preparation amended the compositions and differential abundance of intestinal bacteria of the rats to increase some beneficial bacteria like Lactobacillus, Bacillus, Hungatella, and Romboutsia, and to decrease some potentially harmful genera such as Bacteroides, Escherichia-Shigella, and Proteus. Generally, BaciMix preparation displayed helpful effects on the AAD rat model.
INTRODUCTION
Bacillus is present in soil, air, fermented foods, and the human gastrointestinal tract [1]. In addition to lactic acid bacteria, Bacillus is another bacterial group that is often employed as a probiotic for both people and animals due to its valuable properties such as health promotion, immune stimulation, and disease prevention [2, 3]. Bacillus displays some beneficial effects such as favoring advantageous colonizing conditions for friendly bacteria, immune-stimulating, inhibiting pathogens, and secreting helpful substances in food digestion [3]. Bacillus spores can withstand extremely hard environmental conditions as well as dramatic changes in the living environment. Among this genus, several Bacillus species such as B. subtilis, B. coagulans, B. clausii, B. amyloliquefaciens, B. licheniformis, and B. pumilus have become probiotic bacteria used for humans and animals [1, 3, 4].
Antibiotic-associated diarrhea (AAD) is defined as diarrhea caused only by antibiotic usage, which was orally administrated. Antibiotic overuse in therapy disrupts the gut microbiota balance, altering the body's immunological responses [5]. The usage of cefoperazone lowers short-chain fatty acid (SCFA) concentrations [6], which results in a decrease in proinflammatory cytokines production like TNF-a, IL-6, and IL-12 and an increase in the production of anti-inflammatory cytokines IL-10 and IL-12 [7]. Besides the changes in immune indices, AAD also alters the intestinal microbiota, causing microorganism imbalance in the host organisms.
According to FAO/WHO, pharmaceuticals in general and probiotics in particular should be tested on animal models before use for humans [8]. Among them, rats are often employed due to the physiological similarity between them and humans, and the suitable size for handling and performing experiments [9]. There are many Bacillus probiotic products such as MegaSporeBioticTM, Bacillus licheniformis Zhengchangsheng® that show beneficial effects on the host’s organisms [4, 10]. After testing for acute and semi-chronic toxicity in mice and rats [11], BaciMix preparation, containing Bacillus subtilis BS 304.04 and Bacillus coagulans BC 304.06, was used in this study to evaluate the effects of BaciMix preparation on general health assessments, some immune indices, including IgA in sera and intestinal mucosae as well as IL-6 and TNF-α. Additionally, the intestinal microbiota of the AAD rats was examined.
MATERIALS AND METHODS
BaciMix preparation
BaciMix preparation with a density of 3 × 109 CFU/g for each strain, packaged 1g in each vial, was produced at the GMP-certified factory of Nam Viet Biotechnology Joint Stock Company, addressed at A3-A4 Lot, Small and Medium-sized Industrial Cluster, Dien Phu Commune, Dien Khanh District, Khanh Hoa Province. The BaciMix preparation coded 0121DL was manufactured on January 10, 2022, with expiration date of January 01, 2024. The time for quality management of the BaciMix preparation was approximate 10 days.
Animals
A total of 32 male and female Wistar rats with a weight of 180 ± 20 g were provided by the Military Medical University (Hanoi, Vietnam). The rats were kept at the Military Medical Academy's Experimental Animal Center in the condition of 23 ± 2°C and 50-80% relative humidity and had free access to food and water. The experimental tests for rats were authorized by the Laboratory Animal Welfare and Ethics Committee of the Institute of Microbiology and Biotechnology, Vietnam National University (license number 03:2021/VNU-IMBT). All experimental techniques and animal care were carried out according to the rules established by the Vietnamese Laboratory Animal Welfare and Ethics Committee.
Lincomycin, manufactured on September 25, 2021, with the registration code VD-29184-18, and an expiration date of September 25, 2024 (Domesco, Vietnam), was used for inducing diarrhea in the rats.
Experiment design
After accommodating for 5 days, 32 rats were randomly divided into 4 groups with 8 rats for each group as follows: i) the control group (TCG) received distilled water of 0.1 ml/100g/24h; ii) antibiotic-associated diarrhea group (AAD) was dosed with lyncomicin of 5 mg/kg/24h for 4 days before getting their blood and ceca; iii) the self-healing group (THG) was received lyncomicin at the dose of 5 mg/kg/24h for 4 days before receiving distilled water of 0.1 ml/100g/24h for 5 days; iv) BaciMix group (BAG) was received lyncomicin of 5 mg/kg/24h for 4 days, and then dosed with the BaciMix preparation at the dosage of 1.68 × 109 CFU/kg/24h for 5 days [11]. The rats were then executed by using diethyl ether, and their blood and ceca were dissected to evaluate IL-6, TNF-a, and IgA in sera and intestinal mucosae. The ceca were stored at -80oC until the time to extract the total bacterial DNA.
General assessments of animals
The diarrhea scores and the body weights of the rats were examined every day during the experimental time. Diarrhea scoring was based on the following criteria: i) 0 points: for healthy rats; ii) 1 point: for rats displaying a normal mental state, accompanied by loose and non-adherent perianal stools; iii) 2 points: for rats displaying a bad mental state, adhesion stool around the anus, inappetence, and weight loss [12].
Histopathological analysis
The liver, kidney, spleen, and cecum of the rats were examined at the end of the experiment by weighing and observing under a microscope. The cecal specimens were fixed in 10% formalin for microscopic observation. A standard ruler according to each objective 4X, 10X, and 20X was used to measure the thickness of the cecum mucosa. The mucosal thickness was estimated from the superficial epithelial cells to the mucosal muscle of the cecum [2].
Cytokine and immunoglobulin analysis
After collection, the blood was allowed to clot for 30 minutes at room temperature before being centrifuged at 3000 rpm/15 minutes. Then, the Rat IL-6 ELISA Kit, Rat TNF-a ELISA Kit, and Rat IgA Uncoated ELISA Kit from Thermo Fisher, USA were used to measure IL-6, TNF-a, and IgA serum via ELISA reactions according to the manufacturer’s instructions. After homogenizing the cecum tissue by Wiggen-D5000 tissue homogenizer (Germany), the mixture was centrifuged at 10,000 rpm for 5 minutes to collect the supernatant to quantify intestinal mucosal IgA as described previously [13].
DNA extraction and sequencing
The QIAamp fast DNA mini stool kit (code 51604, Quiagen, Germany) was used to extract total bacterial DNA from cecal stool samples following the manufacturer’s instructions. After checking for integrity using a 1% agarose gel and determining the concentration using a Nanodrop spectrophotometer (Thermo Fisher, USA), the DNA samples were sent for sequences targeting the V3-V4 regions of the 16S rDNA gene on the Illumina MiSeq system.
Intestinal microbiota analysis
Raw fastq sequence data were denoised by DADA2 software to eliminate chimeras and sequences of unknown length, and the obtained filtered sequences were analyzed using Qiime2 (version 2023.5). A total of 5,965,126 high-quality sequences with an average length of 372.5 bp were subjected to build a phylogenetic tree. The mean read count per sample was 186,410 ± 49,078. Amplicon Sequence Variants (ASVs) with 99% similarity were utilized to generate a feature table and subsequently used to analyze the bacterial composition of each sample by using the Silva database. Prism software (version 9) was utilized to examine alpha-diversity indices including Chao 1, Simpson, Shannon, and Evenness. Beta diversity was analyzed using weighted UniFrac distances, and the results were visualized through Principal Coordinate Analyses (PCoA). Qiime2 was used to do a comprehensive statistical analysis of the bacterial community at both the genus and phylum levels.
Statistical Analysis
All data analyses were conducted using SPSS 26.0 (IBM), and the outcomes were presented as mean values accompanied by their corresponding standard deviation (Mean ± SD). For multi-group analyses, the Krukal Wallis, ANOVA test was used to ascertain statistical significance, while the Wilcoxon test was utilized for pairwise comparisons. Statistical significance was judged for p-values <0.05 or p-values<0.001.
RESULTS
Effect of BaciMix on general characteristics and histopathology
The general health condition and physiological status of the rats were evaluated by diarrhea score, and body weight. The diarrhea status of the rats rapidly increased, getting its peak score on the fourth day and continuing the next day for the BAG group, and even lasting to the sixth day for the THG group (Figure 1). The loose feces and a red inflamed anus were major indicators for successfully inducing AAD in the rats by using Lincomycin [18]. From the first to the fourth experimental day, the body weights of all rats in the three groups (AAD, THG, and BAG) remarkably decreased, and after finishing the Lincomycin administration the weights in both the THG and BAG rat groups increased gradually (Table 1).
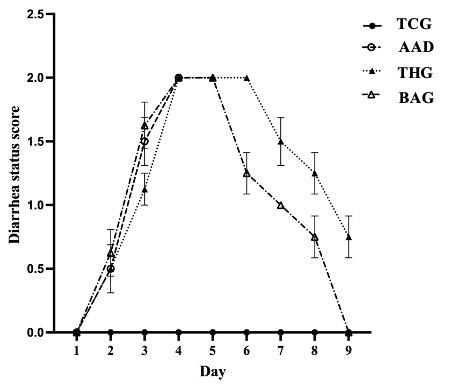
Table 1. Body weight of rats.
The cecum in the rats of the TCG group was normal with tiny and regular nuclei of epithelial cells (Figure 2A). In the BAG group, the tiny and regular nuclei of epithelial cells were also observed, but the capillary stroma was somewhat clogged (Figure 2D). However, in the AAD and THG groups foci of inflammatory cells creating big and tiny lymphoid follicles in the mucosa and submucosa were observed (Figure 2B, 2C). In addition, mild edema and inflammatory infiltrates were also present in both groups. Moreover, in the AAD group, inflammatory lesions promoted surface gland degeneration, and many of them were atrophied.

Effect of BaciMix on inflammatory cytokines and immunoglobulin
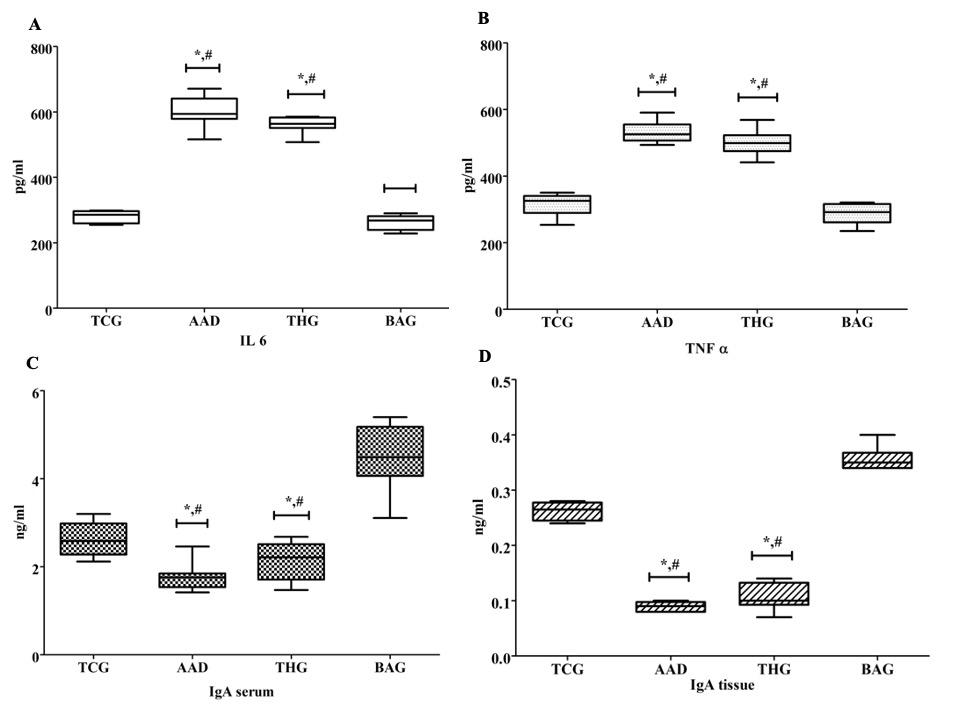
IL-6 and TNF-α levels of the AAD and THG groups were higher than those in the TCG and BAG groups, and these differences were statistically significant (p <0.05) (Figure 3A, 3B). In contrast to cytokines, IgA concentrations in the sera and intestinal mucosae of the AAD and THG groups declined considerably compared to those of the BAG and TCG groups (p<0.05) (Figure 3C, 3D).
Effect of BaciMix on intestinal bacterial diversity in the AAD rats
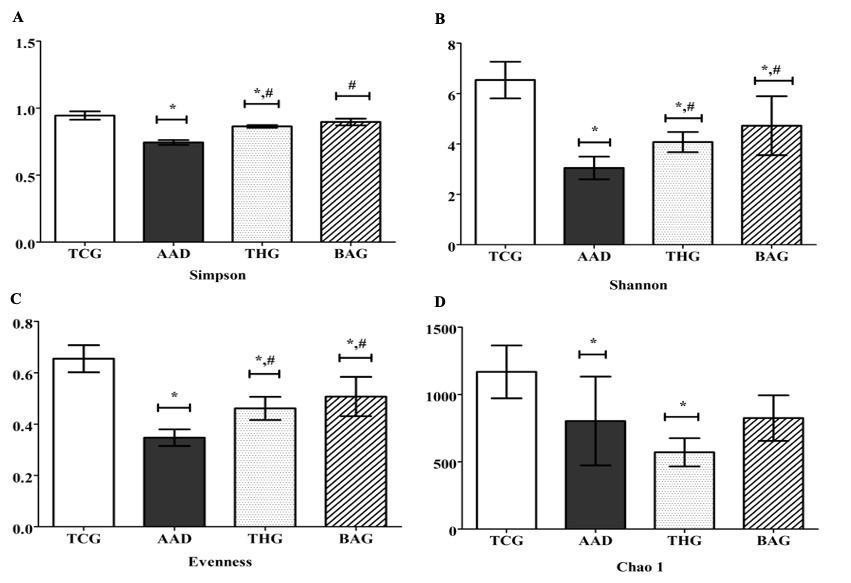
The intestinal bacterial diversity of the rats was examined for alpha diversity and beta diversity. The data were compared between the BAG group and the AAD and THG groups as well as between the BAG group and the TCG group. For alpha diversity, the Simpson, Shannon, Evenness, and Chao 1 indices were evaluated. Except Chao 1, the three remaining indices, including Shannon, Simpson, and Evenness, showed significant differences between the BAG and the AAD groups (p<0.05) (Figure 4). However, for all four indices, there were no significant differences between the BAG and THG groups (p>0.05), but significant differences were observed between the BAG and the TCG groups for Shannon and Evenness indices (p<0.05).
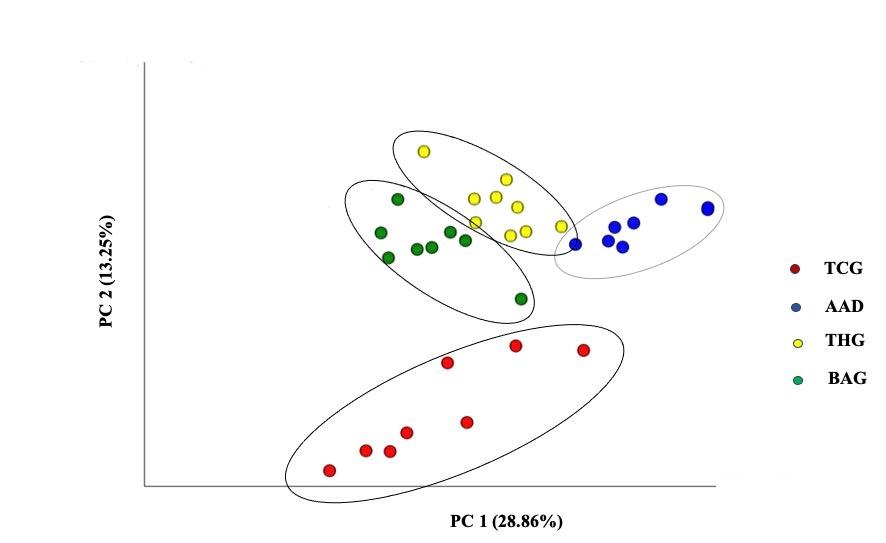
For beta diversity, the PCoA showed that the TCG group was distinct from the three remaining groups, i.e., the groups of AAD, THG, and BAG, and among them, the BAG group was separate from the AAD and THG groups. The variances of all the samples determined by PC1 and PC2 were 28.86%, and 13.25%, respectively (Figure 5).
Effect of BaciMix on phylum and genus compositions of the intestinal microbiota
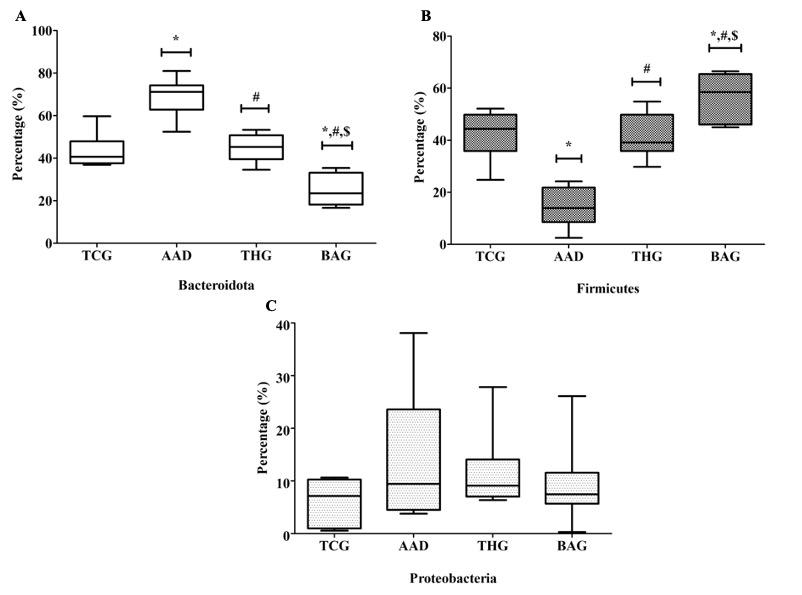
Similar to intestinal bacterial diversity, the abundance of phylum and genus compositions of the gut microbiota was compared between the BAG group and the AAD and THG groups as well as between the BAG group and the TCG group. Bacteroidota, firmicutes, and proteobacteria were the most dominant phyla in all the rat groups. In comparison with the BAG and TCG groups, bacteroidota (Figure 6A) and Proteobacteria (Figure 6C) abundance was the highest in the AAD group, and Bacteroidota showed a significant difference (p<0.05). In contrast to Bacteroidota and Proteobacteria, Firmicutes (Figure 6B) abundance was the lowest in the AAD group, and there was a significant difference with those in the TCG and BAG groups (p<0.05) (Figure 6). In addition, the differences of Bacteroidota and Firmicutes abundance were significant between the BAG and THG groups, and between the BAG and TCG groups (p<0.05).
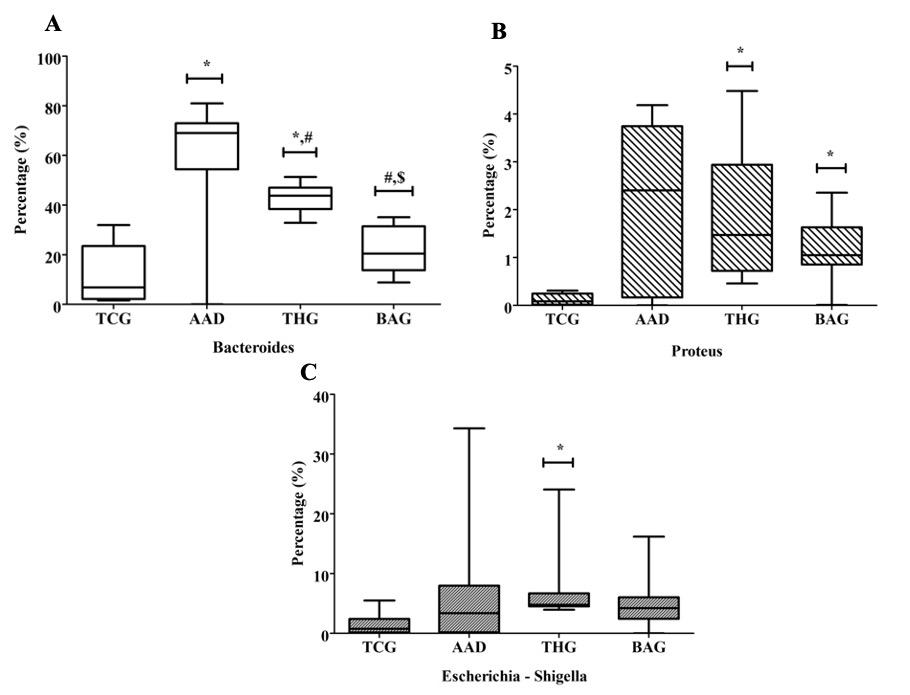
At the genus level, the differential abundance of some harmful and beneficial/natural bacteria was compared for all the groups (Figure 7). In comparison with the AAD and THG groups, Bacteroides (Figure 7A), Proteus (Figure 7B), and Escherichia – Shigella (Figure 7C) abundance in the BAG group was the lowest, and among them significant difference was observed for Bacteroides (p<0.05). However, in comparison with the TCG group, Proteus abundance in the BAG group showed significant difference (p<0.05).
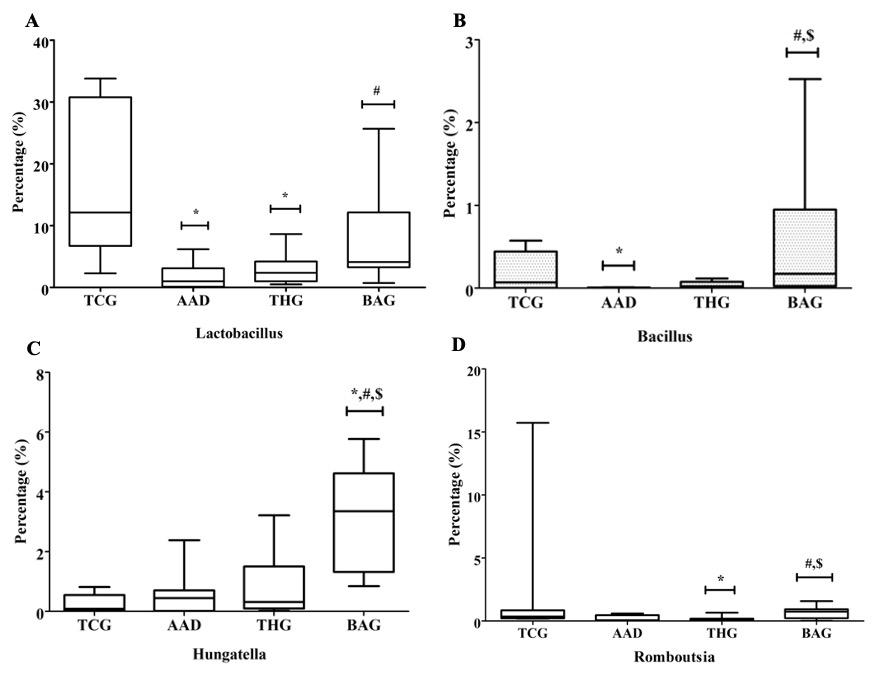
In contrast to harmful bacterial genera, some beneficial bacteria significantly increased in the BAG group compared with the AAD and THG groups. In comparison with the AAD and THG groups, Lactobacillus (Figure 8A), Bacillus (Figure 8B), Hungatella (Figure 8C), and Romboutsia (Figure 8D) abundance in the BAG group was the highest, and there were significant differences for Bacillus, Hungatella, and Romboutsia (p<0.05). Among the beneficial bacteria, significant differences were observed between the BAG and AAD groups for Lactobacillus and between the BAG and TCG groups only for Hungatella (p<0.05).
DISCUSSION
Probiotics have offered many beneficial effects such as favoring advantageous colonizing conditions for friendly bacteria, immune-stimulating, inhibiting pathogens, and secreting helpful substances in food digestion for host organisms [3]. Besides Lactic Acid Bacteria, Bacillus is often employed as a probiotic for both people and animals due to its valuable properties. In this study, BaciMix preparation, a combination of Bacillus subtilis BS304.04 and Bacillus coagulans BC 304.06, was used to evaluate its alleviated effects on the AAD rat model. The parameters used for assessment were general conditions (AAD score, body weight, histopathological analysis, and cecum thickness), some immune indices (IgA in sera and mucosae, as well as IL-6 and TNF-α), and intestinal microbiota changes in the AAD rats.
The general conditions, including AAD score, body weight, histopathological analysis, and cecum thickness, were improved in the AAD rats treated by BaciMix preparation but there were no significant differences among all the groups (p > 0.05). These general indicators were supported by the analytical result of some immunological indices such as IgA in the sera and intestinal mucosae, as well as IL-6 and TNF-α. Many studies have shown that probiotics' immunomodulatory effects might occur directly by stimulating macrophages or natural killer cells, indirectly by modulating the synthesis of cytokines or immunoglobulins, or both [13, 14]. This could further improve intestinal barrier function, modify the makeup of the intestinal microbiota, or regulate intestinal metabolites [14, 15]. In our study, the amounts of pre-inflammatory cytokines, including IL-6, and TNF-α significantly decreased in the BAG group compared with the AAD and THG groups. The decrease in the IL-6 and TNF-α levels in the BAG group might indicate that the BaciMix preparation could not be a factor which triggered intestinal inflammation. In contrast to pre-inflammatory cytokines, IgA concentrations in the rats’ sera and intestinal mucosae significantly increased in the BAG group compared with the AAD and THG groups. IgA concentrations in the rats’ sera and intestinal mucosae in the BAG group would indicate that BaciMix preparation can lower intestinal inflammation and enhance local immune response. Our results were consistent with those in the research of Deng et al. (2017) and Neag et al. (2020), which showed that the probiotics, composed of Bacillus spp, decreased proinflammatory cytokines (TNF-α, IL-1β) [4, 16]. In addition, the probiotic mixture, composed of Lactobacillus and Bifidobacterium species in the research of Karamese et al. (2016), decreased the levels of TNF-α, IL-6, and TGF-β, and increased the levels of IL-10, IgG, and IgA in the serum [13].
In addition to some general health indicators and immune indices, the alteration of the gut microbiota of the rats was examined. Gut microbiota occupies for over 70% of the total microbiota and contributes to many reactions to protect the body from potentially harmful factors [17]. The intestinal bacterial compositions of the rats were altered after using Lyncomycin and the BaciMix preparation. The alteration was evaluated for alpha and beta diversity as well as differential abundance of some phyla and genera of all four groups. In the BAG group, the relative abundance of the Bacteroidetes and Proteobacteria notably decreased compared with the AAD and THG groups in the current study. Both Bacteroidetes and Proteobacteria are composed of a variety of pathogens associated with biological problems and illnesses such as inflammatory illnesses (IBD), obesity, and HIV [18, 19]. In contrast to Bacteroidetes and Proteobacteria, Firmicutes, a common bacterial phylum in the gut of healthy people and decreasing during disease, significantly increased in the BAG group compared with the three remaining groups, i.e., TCG, ADD and THG groups. Similar to phylum level, at the genus level some useful changes were also observed. In particular, some beneficial bacterial genera, including Lactobacillus, Bacillus, Hungatella, and Romboutsia, remarkably increased in AAD rats treated by BaciMix preparation. In addition, BaciMix preparation decreased the number of potentially dangerous bacteria such as Bacteroides, Proteus, and Escherichia – Shigella. Interestingly, most of these changes were of significant differences in comparison between AAD rats treated by BaciMix preparation (BAG group) and untreated AAD rats, i.e., the AAD and THG groups. Moreover, the differences between the BAG group (AAD rats treated by BaciMix preparation) and the TCG group (control rats) were milder and not significant. We know that Bacteroides have been linked to clinical anaerobic infections [20], inflammatory bowel disease [21], and bacteremia [22]; Shigella sp. is the most common pathogen linked with infectious diarrhea [23], and Proteus is an opportunistic pathogen; whereas Lactobacillus and Bacillus have shown extensively useful for health benefits. Thus, through the administration of the multi-strain probiotic BaciMix preparation, the intestinal microbiota of the AAD rats was altered by biasing to beneficial effects for health.
Briefly, the use of Lincomycin changes some immune indices and intestinal microbiota in the AAD rats in comparison with the healthy rats. The AAD rats treated by BaciMix preparation underwent milder effects caused by antibiotics compared with untreated ADD rats. Additionally, the data, including immune indices (TNF-α, IL-6, IgA in sera and intestinal mucosae) and microbiota, of the AAD rats treated by BaciMix preparation were closer to those of the healthy rats. These data were supported by the results of the general assessment and histopathological analysis that showed AAD conditions of the BAG and TCG rat groups were better than those in the AAD and THG groups. Generally, our data were accordant to demonstrate that BaciMix preparation showed positive effects on the AAD rat model.
CONCLUSIONS
The results of this study showed that the BaciMix preparation remarkably reduced the effects of the antibiotic regarding general assessments (diarrhea score, cecum thickness), specific immune indices (IL-6, TNF-α, and IgA in sera and intestinal mucosae), and amplicon metagenomic analysis (the V3-V4 sequencing region of 16S rDNA gene). In general, the use of multi-strain probiotic BaciMix preparation exhibited helpful effects in improving the health status of the AAD rat model.
ACKNOWLEDGMENTS
The authors would like to acknowledge the grant “Evaluation of intestinal microflora improvement and immunity enhancement of multi-strain probiotic products” (Number ĐTĐL.CN-61/19) funded by the Ministry of Science and Technology, Vietnam.
AUTHOR CONTRIBUTIONS
DHN developed the ideas, designed the study, and conceptualized the manuscript; all authors collected the data, analyzed the data; DHN, TSN, DTC, QUN and HVV drafted the manuscripts; DHN and QUN revised and edited the manuscript; HVV supervised. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Sorokulova I. Modern status and perspectives of bacillus bacteria as probiotics. Journal of Probiotics & Health. 2013;1:1-5.
- [2]Afrin M, Sachi M, et al. Evaluation of optimum dietary inclusion level of probiotics for potential benefits on intestinal histomorphometry, microbiota, and ph in japanese quails. Journal of Advanced Biotechnology and Experimental Therapeutics. 2021;4.
- [3]Luise D, Bosi P, et al. Bacillus spp. Probiotic strains as a potential tool for limiting the use of antibiotics, and improving the growth and health of pigs and chickens. Frontiers in Microbiology. 2022;13.
- [4]Neag MA, Catinean A, et al. Probiotic Bacillus spores protect against acetaminophen induced acute liver injury in rats. Nutrients. 2020;12:632.
- [5]Willing BP, Russell SL, et al. Shifting the balance: Antibiotic effects on host–microbiota mutualism. Nature Reviews Microbiology. 2011;9:233-43.
- [6]Theriot CM, Koenigsknecht MJ, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nature Communications. 2014;5:3114.
- [7]Säemann MD, Böhmig GA, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of il-12 and up-regulation of il-10 production. Faseb j. 2000;14:2380-2.
- [8]Joint F. Who working group report on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada. 2002;30:16-22.
- [9]Iannaccone PM and Jacob HJ. Rats! Dis Model Mech. 2009;2:206-10.
- [10]Lu X, Jing Y, et al. Bacillus licheniformis zhengchangsheng® inhibits obesity by regulating the amp-activated protein kinase signaling pathway. Probiotics Antimicrob Proteins. 2021;13:1658-67.
- [11]Nguyen DH, Ta TNA, et al. The acute toxicity and semi-chronic toxicity of bacimix product containing two strains of Bacillus subtilis BS 304.04 and Bacillus coagulans BC 304.06 on experimental animal model. Journal of Military Pharmaco-medicine. 2022;47:133-44.
- [12]Ren DD, Li SS, et al. Panax quinquefolius polysaccharides ameliorate antibiotic-associated diarrhoea induced by lincomycin hydrochloride in rats via the mapk signaling pathways. J Immunol Res. 2022;2022:4126273.
- [13]Karamese M, Aydin H, et al. The immunostimulatory effect of lactic acid bacteria in a rat model. Iran J Immunol. 2016;13:220-8.
- [14]La Fata G, Weber P, et al. Probiotics and the gut immune system: Indirect regulation. Probiotics and antimicrobial proteins. 2018;10:11-21.
- [15]Sanders ME, Merenstein DJ, et al. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nature reviews Gastroenterology & hepatology. 2019;16:605-16.
- [16]Deng B, Wu J, et al. Probiotics and probiotic metabolic product improved intestinal function and ameliorated lps-induced injury in rats. Curr Microbiol. 2017;74:1306-15.
- [17]Ho JT, Chan GC, et al. Systemic effects of gut microbiota and its relationship with disease and modulation. BMC immunology. 2015;16:1-6.
- [18]Hand TW, Vujkovic-Cvijin I, et al. Linking the microbiota, chronic disease, and the immune system. Trends in Endocrinology & Metabolism. 2016;27:831-43.
- [19]Zhao L. The gut microbiota and obesity: From correlation to causality. Nature Reviews Microbiology. 2013;11:639-47.
- [20]Elliott D, Kufera JA, et al. The microbiology of necrotizing soft tissue infections. Am J Surg. 2000;179:361-6.
- [21]Wu S, Powell J, et al. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappab pathway. Infect Immun. 2004;72:5832-9.
- [22]Brook I. Clinical review: Bacteremia caused by anaerobic bacteria in children. Crit Care. 2002;6:205-11.
- [23]Bona M, Medeiros PH, et al. Virulence-related genes are associated with clinical and nutritional outcomes of shigella/enteroinvasive Escherichia coli pathotype infection in children from brazilian semiarid region: A community case-control study. Int J Med Microbiol. 2019;309:151-8.