Exploring the role of autoantibodies in Iraqi females with polycystic ovary syndrome
Abstract
Polycystic ovary syndrome (PCOS) is a prevalent endocrinopathy that affects 5-10% of women during their reproductive age. Recent research suggests a potential link between autoantibodies and PCOS development. The current study aimed to investigate the relationship between autoantibodies and PCOS. One hundred and fifty female patients suffered from PCOS aged between 20 and 35 years participated in this study as matched with healthy control. The result showed a significantly higher concentrations of anti-thyroid peroxidase (TPO) and anti-thyroglobulin (TG) autoantibodies in the patients compared to the healthy control group. Moreover, the concentrations of IgM and IgG for anti-cardiolipin (ACL) were significantly higher in the patients than in the control group. These increments in ACL antibodies in patients suggested a risk factor for PCOS. Conversely, no significant correlation was found in case of β-2 glycoprotein I (β2GPI) antibodies and lupus in patients. Serum amyloid A (SAA) and high-sensitivity C-reactive protein (hs-CRP) were significantly higher in patients. Procalcitonin (PCT) was also highly significant in patients compared to the controls. Lastly, the concentration of total IgG antibodies was significantly higher in patients than in controls. In conclusion, high concentrations of autoantibodies in patients suggest a risk factor and positive associations with PCOS in females.
INTRODUCTION
Polycystic ovarian syndrome (PCOS) demonstrates the most prevalent endocrine condition affecting women. It often leads to irregular menstruation and infertility in women of reproductive age. Hormonal and genetic factors both participate in the development of PCOS [1]. The clinical symptoms of PCOS include hyperandrogenism, disrupted ovulation, polycystic ovaries, and metabolic irregularities such as insulin resistance and dyslipidemia [2]. Recent research findings suggest a plausible correlation between autoimmune mechanisms and the development of PCOS [3]. Moreover, according to a study, there may be a correlation between PCOS and the development of autoimmune mechanisms, sparking a growing interest in investigating the potential involvement of autoimmunity in the pathogenesis of PCOS [4]. Additionally, an increasing incidence of PCOS has been associated with several diseases, like diabetes mellitus, thyroid disorders, and psoriasis [4]. Some researchers suggest that PCOS is not an autoimmune disease but rather than endocrine condition, a positive association exists between PCOS and anti-nuclear antibodies (ANA) [5, 6]. Evidence suggests that ANA and anti-thyroid peroxidase (anti-TPO) autoantibodies could influence the long-term clinical strategies for patients health [7, 8]. Some findings indicate that there are positive associations between PCOS and inflammation indicators such as serum amyloid A (SAA), high-sensitivity C-reactive protein (hs-CRP), procalcitonin (PCT) [9, 10]. In autoimmune thyroiditis, higher levels of antibodies triggered against the self-tissue of thyroid glands such as anti-thyroid peroxidase (TPO), thyrotrophic receptor (TRAbs), and thyroglobulin (TG) antibodies [11-13]. This humoral immune response expands the role of crazy T-cells and massive activity against self-antigens [14]. Several studies have reported higher levels of TPO and TG autoantibodies in women with PCOS [15, 16].
Moreover, growing evidence suggests that common diagnostic tests for anticardiolipin antibodies (ACL) and lupus anticoagulants (LAC) help to diagnose antiphospholipid syndrome (APS)-associated POCS [17, 18]. However, monoclonal or polyclonal autoantibodies, such as anti-phosphatidylserine antibodies and ACL, can specifically destroy trophoblasts, inhibit syncytium formation, suppress human chorionic gonadotropin production, and prevent trophoblast invasion in both in vitro experimental models and animal studies [19]. ACL and the anti-phosphatidylserine antibody frequently react with one another. An anti-phosphatidylethanolamine antibody (aPE) is strongly linked to very early POCS [20, 21]. In POCS patients, hormonal imbalances might cause alterations in B cell frequencies similar to those seen in females. This effect was inhibited by treating patients with rising levels of natural antibodies while administering a medication that inhibits the function of male sex hormones [22, 23].
The current study aimed to investigate the frequency of these autoantibodies among individuals diagnosed with PCOS to shed light on their potential role in this syndrome’s etiology and clinical manifestations.
MATERIALS AND METHODS
Study population
This study explored the potential relationship between autoantibodies and PCOS in female patients. This study involved 150 women aged 20 to 35 with a clinical diagnosis of PCOS in Baghdad City, Iraq. Additionally, a control group of 150 healthy individuals matched in age to the patient group was included. Autoantibodies targeting TPO, TG, cardiolipin IgM and IgG, β-2 glycoprotein I (β2GPI) (IgM and IgG), as well as other total antibodies Ig (M, A, and G), were evaluated in both the patient and control groups. Further, SAA, hs-CRP, and PCT were also tested. Women with multiple health conditions, such as uterine abnormalities, endocrine irregularities, or diabetes mellitus, were excluded from the study. This study was approved by Committee of Scientific Research Ethics, Amara Medical Institute under the administrative order number, 7/18/5 in 4/1/2023.
Study sampling
The study was conducted from January to May 2023. Venous blood specimens were collected from each participant using plain tubes with a total volume of milliliters. Subsequently, the samples underwent centrifugation to separate the serum fraction, which was then subjected to laboratory analysis to determine the serum concentrations of TPO, TG, cardiolipin (IgM and IgG), β2GPI (IgM and IgG), as well as other antibodies (IgM, A, and G). Also, SAA, hs-CRP, and PCT were measured.
Estimation of autoantibodies
The concentrations of autoantibodies in serum of participants for TG and TPO were quantified using luminescence immunoassay (CLIA) methods with the MAGLUMI® X3 instrument and kit from MAGLUMI® X3 (Diagnostics GmbH, Sandhofer Strasse 116; D-68305 Mannheim, 2020).
Serum specimens were collected from participants for the detection of autoantibody levels against β2GPIand ACL using enzyme-linked immunosorbent assay (ELISA) methods by BioTek 50-TS instrument and kits from BioSource (MBS164474, MBS580079 kits, USA, San Diego, CA 92195-3308, 2019).
The screening for lupus was conducted using STAGO Vitro-Diagnostics industry equipment and kit from STAGO (USA, ST 2258 D, 2020) following the guidelines outlined by the International Society on Thrombosis and Haemostasis.
The serum levels of IgM, IgA, and IgG antibodies were determined using nephelometry methods with the PA45 specific protein analyzer from Genrui-Biotech Inc. (Geya Technology Park, Guangming District, 518106, Shenzhen, China).
Estimation of SAA, hs-CRP, and PCT
The serum levels of SAA, hs-CRP, and PCT were determined using nephelometry methods with the PA45-specific protein analyzer from Genrui-Biotech Inc. (Geya Technology Park, Guangming District, Anti-SAA No KIT (E6657), PCT NO KIT (U7789), Hs-CRP No KIT (D6678), Shenzhen, China).
Statistical analysis
Descriptive statistics, including means, standard errors (SE), and frequencies, were calculated by GraphPad Prism 8.0.02. Also, comparative analyses such as T-tests and risk factor were used to estimate the difference between the groups.
RESULTS
In this section, the findings obtained from investigating various parameters in PCOS patients, including all autoantibodies and markers such as TPO, TG, ACL IgM, ACL IgG, β2GPI IgM, β2GPI IgG, lupus, IgM, IgM, IgM, IgA, IgG, SAA, hs-CRP, and PCT were explained.
Anti-thyroid antibodies in PCOS patients
Table 1 presented the relationship and risk ratio (RR) of anti-thyroid antibodies in PCOS patients. The study showed a substantial rise in the RR for anti-TPO (RR=6.4), which strongly links this antibody to PCOS (Table 1). The study also found an increase in the RR for another antibody, anti-TG (RR=5.4). These data indicates that when these antibodies, TPO and TG, were present at high levels, which may increase the risk of developing PCOS.
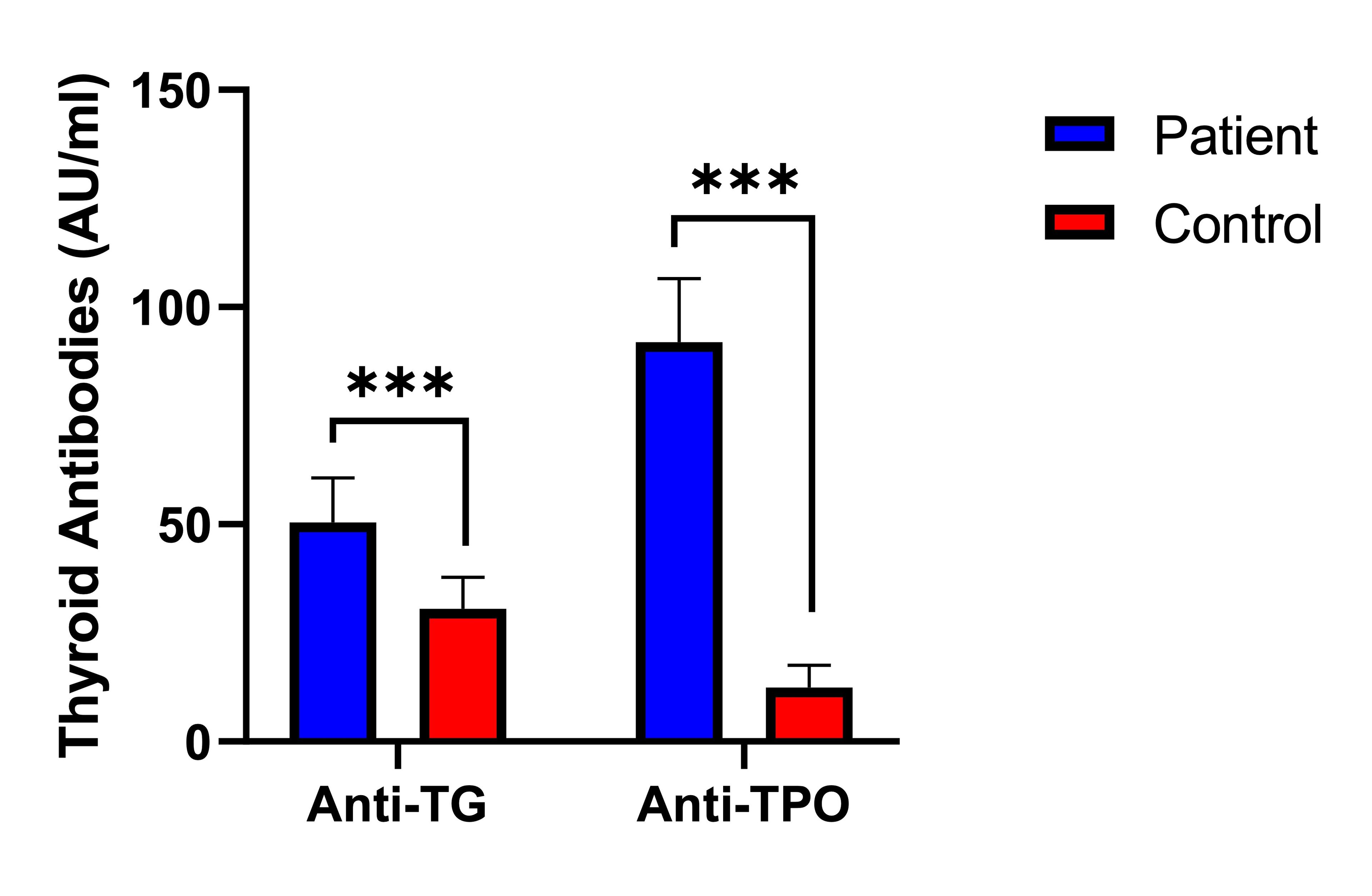
Figure 1 showed a significant difference (p<0.001) in the serum levels of antibodies against TPO and TG between the subject group and the control. The TPO antibody concentrations were markedly higher in PCOS patients (91.9±14.7 AU/ml) compared to the controls (12.4±5.1 AU/ml). Similarly, the serum levels of TG antibodies within the subjects (50.4±10.3 AU/ml) were significantly higher compared to those in the control (30.6±7.2 AU/ml). These findings emphasized the notable difference in antibody levels between individuals diagnosed with PCOS and those in the control.
Table 1. Concentration and risk ratio (RR) for antithyroid antibodies in PCOS patients
Cardiolipin, β2-glycoprotein I, and lupus in PCOS patients
Table 2 presented the levels of IgM and IgG for ACL, and β2GPI, lupus concentrations, and their respective detection risk ratios (RR) in the patients. The findings indicate a significant difference (p<0.001) in ACL antibody levels between patients and the controls. The ACL-associated IgM levels were significantly higher in patients (19.2±4.2 AU/ml) compared to the controls (3.3±1.0 AU/ml). Similarly, the ACL-associated IgG levels were notably elevated in patients (22.5±2.3 AU/ml) relative to the control (2.9±1.2 AU/ml). The detection of RR for IgM and IgG with values of 4.2 and 5.6, respectively, showed a positive correlation with the disease, implying a strong association between the increased presence of ACL autoantibodies and PCOS.
Conversely, no significant correlation was found in case of β2GPI antibodies and lupus in patients compared to controls (Table 2). The detection of RR further supports the understanding of their correlation with PCOS development.
Table 2. IgM and IgG levels for ACL, β2GPI, and concentration of Lupus in patients
Inflammatory marker in PCOS patients
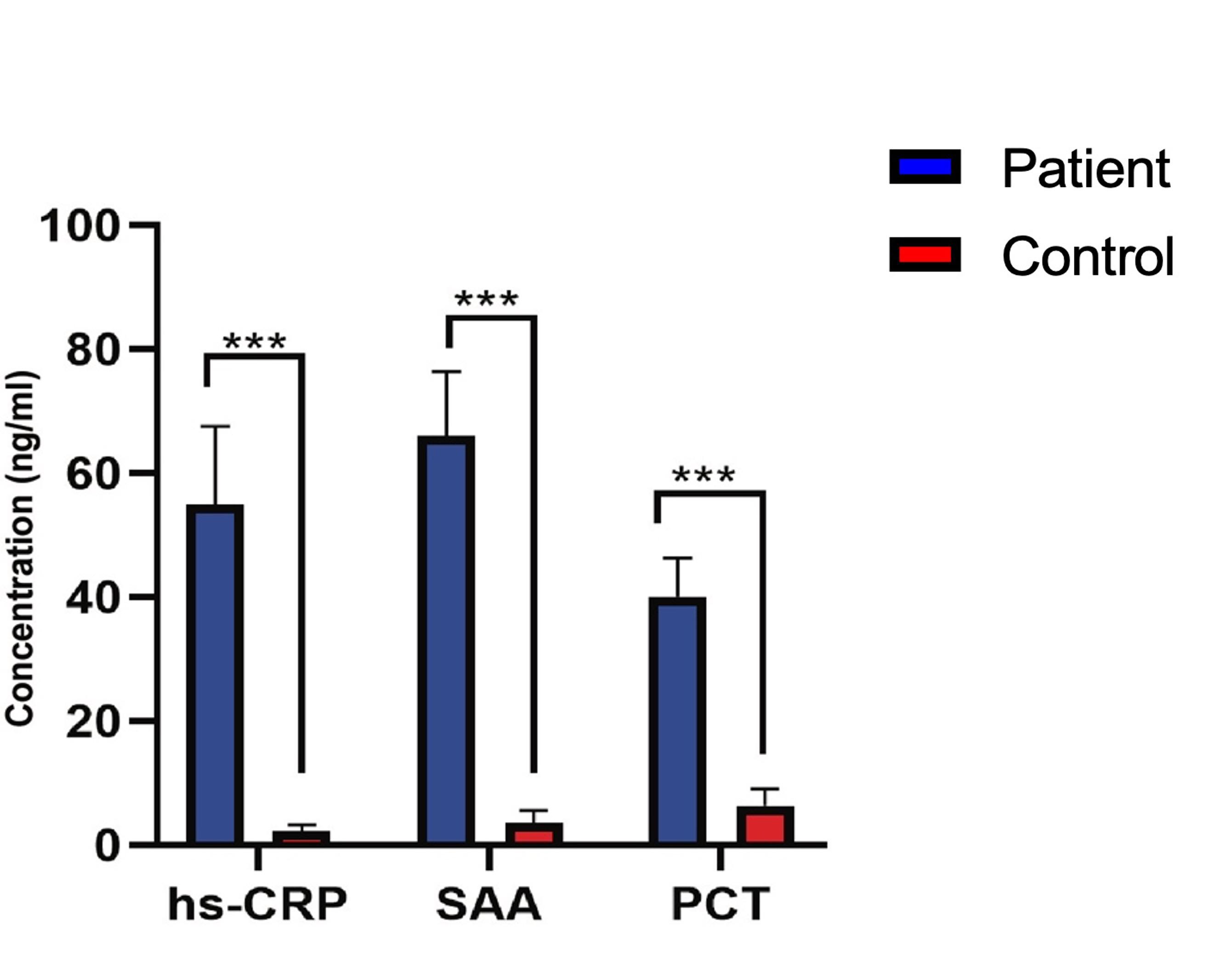
Table 3 stated the significant insights on specific inflammatory biomarkers in PCOS patients compared to the controls. Initially, the concentration of hs-CRP was noticeably elevated in PCOS patients (55± 12.5 ng/ml) relative to the control group (2.3 ± 0.98 ng/ml), suggesting a strong correlation of hs-CRP with PCOS. Similarly, SAA levels were significantly increased in PCOS patients (66 ± 10.4 ng/ml) compared to the control group (3.6 ± 1.9 ng/ml), displaying a substantial RR value of 7.6. Moreover, PCT concentrations were higher in PCOS patients (40 ± 6.3 ng/ml) compared to the control group (6.4 ± 2.6 ng/ml), with a noteworthy RR value of 4.2. These observations imply that hs-CRP, SAA, and PCT could function as biomarkers for identifying and evaluating PCOS risk in female.
Figure 2 depicts a comparison of inflammatory biomarker levels between patient and controls. It showed significantly elevated levels of hs-CRP, SAA, and PCT in patients compared to controls. Patients had approximately 6 times higher hs-CRP levels, 7 times higher SAA levels, and over 4 times higher PCT levels, indicating an ongoing inflammatory process in the PCOS patients. The tight confidence intervals around the relative risk suggest a high degree of certainty in these results.
Table 3. Comparison of inflammatory biomarker levels in patients with PCOS
Immunoglobulins in PCOS patients
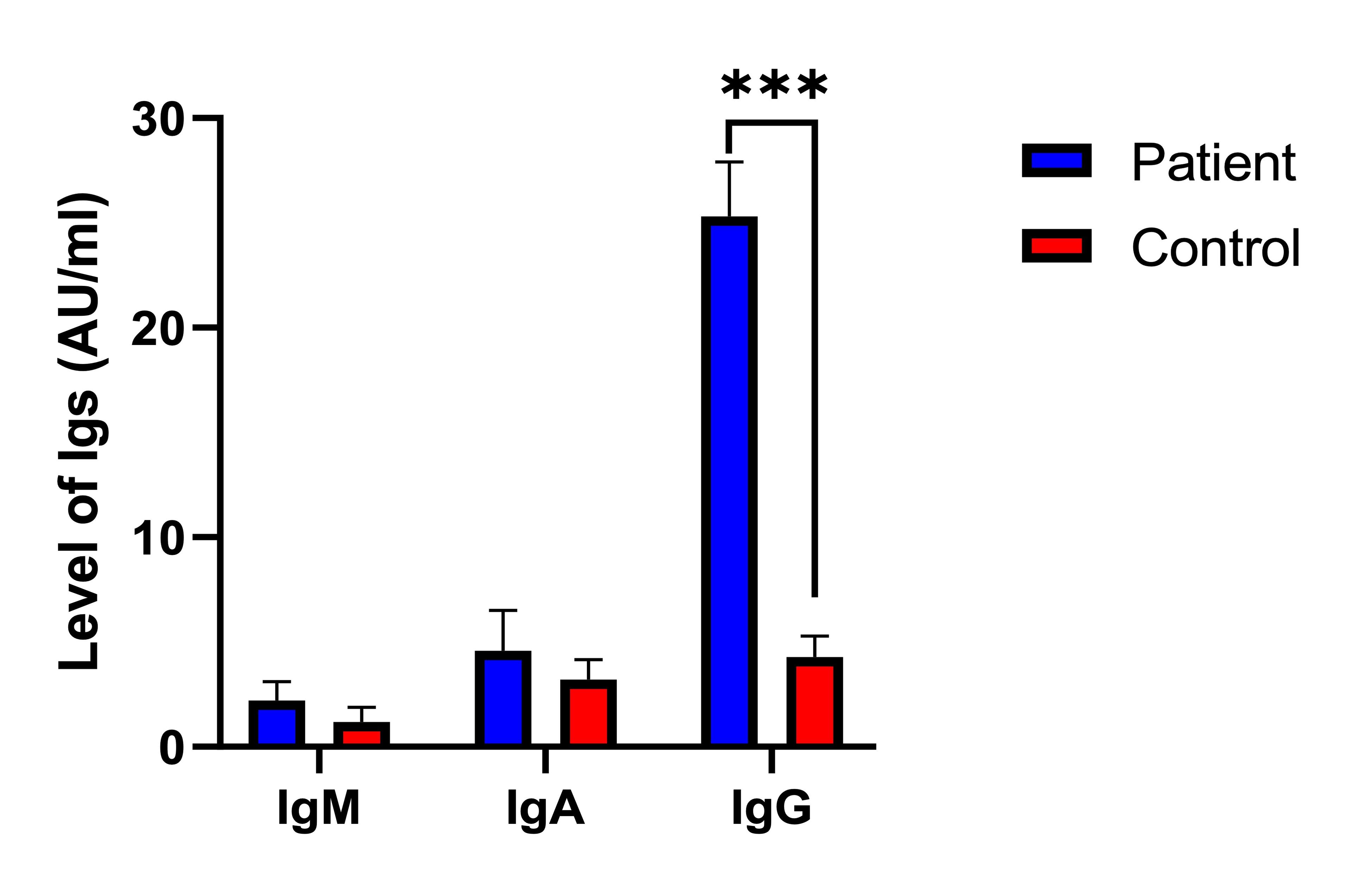
Table 4 revealed the levels of total antibodies, specifically IgM, IgA, and IgG in patients with PCOS compared to the control. No significant difference was observed in the IgM antibody levels between PCOS patients (2.2±0.92 AU/ml) and the control (1.2±0.69 AU/ml), indicating no strong association between IgM levels and PCOS. Similarly, the levels of IgA antibodies did not significantly differ between PCOS patients (4.6±1.9 AU/ml) and the control (3.2±0.96 AU/ml), suggesting no significant association between IgA levels and PCOS. In contrast, the levels of IgG antibodies were significantly higher in PCOS patients (25.3±2.6 AU/ml) than in the control (4.3±1.0 AU/ml), indicating a strong association between elevated IgG levels and PCOS. These findings suggest that while IgM and IgA antibody levels do not significantly correlate with PCOS, elevated IgG levels may be linked to the development or progression of PCOS.
Figure 3 showed the detection risk ratios for total antibodies in patients compared to controls. It displayed the risk ratios of IgM (0.19, 95% CI: 0.99-1.2, P=0.265NS), IgA (0.62, 95% CI: 0.42-0.98, P=0.656NS), and notably high for IgG (2.3, 95% CI: 1.0-2.9, P=0.009). Consistently, the RR explain the total antibody levels between patients and controls, with IgG showing a statistically significant difference.
Table 4. Comparison of total antibody levels in patients with PCOS and control group
DISCUSSION
The current study revealed the high significance of patient women with POCS in the serum of autoantibodies against TPO and TG. It is suggested that these autoantibodies increased in the PCOS women as an autoimmune response. Strong anti-thyroid antibody association with PCOS have been proposed, for instance, anti-TPO 7.81% [21]. Further, anti-TG and anti-TPO antibodies were shown to be strongly associated with PCOS in patients [24]. Another study demonstrated that Anti-TG and anti-TPO antibodies may be related to PCOS, and the higher RR value for thyroid antibodies in patients with PCOS compared to the control [25]. According to a study, PCOS women of reproductive age showed autoimmune thyroiditis three times more frequently than women without PCOS [26]. Infertility, miscarriages, and an abnormal thyroid profile were all strongly correlated among pregnant women. Both hyperthyroidism and hypothyroidism have been linked to a higher prevalence of miscarriages, fetal deaths, and delayed cognitive development in children [27]. However, discrepant findings were reported in individuals with PCOS compared to healthy controls [28]. The variation in results may be attributed to the inclusion of young euthyroid girls presenting with PCOS symptoms in the previous study.
Furthermore, the current research revealed a strong correlation between the presence of anti-TG antibodies and the onset of PCOS. This observation is in line with a previous investigation which documented the existence of these antibodies in 47% of PCOS patients and 41% of healthy females [29]. The results of the current study align with a previous study, which identified anti-TG antibodies in 36% of individuals diagnosed with PCOS and 30% of healthy control participants [28]. However, these findings contradicted those of other researchers who reported anti-TG levels of 113±312 AU/ml in PCOS patients and 4±17 AU/ml in healthy controls. Discrepancies in lifestyle factors, such as obesity, smoking, alcohol consumption, and stress, as well as variations in sample size may contribute to this inconsistency [30-32].
Additionally, the current study revealed a significant and novel finding regarding the association between immunological markers and PCOS. Specifically, we found a strong positive association of IgM and IgG antibodies for ACL in patients compared to the controls. The levels of IgM and IgG antibodies for ACL, β2GPI and the concentration of lupus are accompanied by the corresponding RR values, indicating the detection risk in patients compared to the control. This study alone revealed the positive role of auto-immune complex formation in patients with POCS. There is no previous research explored this aspect, thus, we propose that the elevated ACL levels observed could be attributed to the hormonal imbalances, notably insulin and testosterone dysregulation, inherent in PCOS. Such imbalances are known to have implications on the immune system, potentially culminating in increased ACL levels. Moreover, PCOS is classified as a chronic low-grade inflammatory condition. This persistent inflammation may drive the production of autoantibodies, inclusive of ACL.
Interestingly, a study reported contrasting results, as the patient tested negative for ACL antibodies, aPT (anti-prothrombin antibodies), aPS (anti- phosphatidylserine antibodies), and anti-phosphatidylserine antibodies but exhibited positive results twice for anti-β2G-1(anti β2 glicoprotein-1) antibodies. It was proposed that the presence of anti-β2GP-1 antibodies might be attributed to an infectious agent [33]. However, these findings contradicted the outcomes of the current study.
Also, the current study indicates that women with PCOS often had higher levels of inflammation markers such as hs-CRP, PCT, and SAA. This could be because PCOS is often associated with chronic low-grade inflammation, insulin resistance, and obesity. All these conditions can lead to increased inflammation in the body, which may raise the levels of these markers. Therefore, it is suggested that the higher concentrations of hs-CRP, PCT, and SAA might be associated with PCOS [9, 10, 34-36].
These observations revelated that woman with PCOS had a higher level of IgG. After longer time, mimic antigen may cause chronic immune response as autoimmune antibodies. Hyperandrogenemia is an earmark feature of PCOS that performs a vital role in the pathogenesis and resemble to be immediately related to disease harshness [20, 37]. Although chronic inflammation and altered immune function have been proposed to play a role in the pathogenesis of PCOS, variations in B cell frequencies might be linked to PCOS [38]. It is also suggested that B cells are not major mediator in PCOS, and that androgen receptor activation directly alters their frequency. In addition to having higher levels of circulating IgG, hyperandrogenic women with PCOS also have higher frequencies of age-associated double-negative B memory cells [39, 40].
CONCLUSION
In conclusion, this study has elucidated the significant role of autoantibodies and inflammatory markers in the pathogenesis of PCOS. Notably, increments were observed in autoantibodies against TPO, ACL IgM, and ACL IgG in patients compared to controls. Furthermore, it is noted the elevated levels of IgG alongside significant elevations in inflammatory markers, such as hs-CRP, SAA, and PCT in PCOS patients. These findings suggest a strong positive association between these increased levels of autoantibodies and PCOS. The observed variations among different PCOS patients pave the way for future molecular-level research to improve treatment strategies for PCOS patients.
ACKNOWLEDGMENT
The authors appreciate the kind cooperation of the management of Wasit University and, they also thank to Mustansiriyah University, Baghdad, Iraq for its support to this work.
AUTHOR CONTRIBUTIONS
ASAK played a pivotal role in the conceptualization and development of the study’s methodology, carried out all data collection and analysis, wrote the original draft, and was involved in review, editing, and finalization of the manuscript. ASK provided supervision and validation and contributed to review and editing of the manuscript. All authors have approved the final version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Munro MG, Balen AH, et al. The figo ovulatory disorders classification system. Human Reproduction. 2022;37:2446-64.
- [2]Teede HJ, Misso ML, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Human reproduction. 2018;33:1602-18.
- [3]Bajaj S, Gopal N, et al. A pictorial review of ultrasonography of the figo classification for uterine leiomyomas. Abdominal Radiology. 2022:1-11.
- [4]Chahardoli R, Saboor-Yaraghi A-A, et al. Can supplementation with vitamin d modify thyroid autoantibodies (anti-tpo ab, anti-tg ab) and thyroid profile (t3, t4, tsh) in hashimoto’s thyroiditis? A double blind, randomized clinical trial. Hormone and Metabolic Research. 2019;51:296-301.
- [5]Ostrowska L, Gier D, et al. The influence of reducing diets on changes in thyroid parameters in women suffering from obesity and hashimoto’s disease. Nutrients. 2021;13:862.
- [6]Tran VT, Ly LD, et al. Thyroid peroxidase antibodies in infertile women with polycystic ovary syndrome. Reproductive Sciences. 2023:1-6.
- [7]Auda SA, Gatea EA, et al. Evaluation of thyroid autoimmunity markers in polycystic ovarian syndrome in women. Biomedicine. 2023;43:893-6.
- [8]Pan M, Zhang J, et al. Association of serum thyroid hormone levels with androgen and metabolic parameters in chinese women with polycystic ovary syndrome: A retrospective cross-sectional study. Clinical and Experimental Obstetrics & Gynecology. 2023;50:162.
- [9]Rashad NM, El-Shal AS, et al. Association between inflammatory biomarker serum procalcitonin and obesity in women with polycystic ovary syndrome. J Reprod Immunol. 2013;97:232-9.
- [10]Liu H, Meng X, et al. Serum amyloid a in polycystic ovary syndrome. Clin Chim Acta. 2021;518:151-5.
- [11]Fawzy Mohamed Abo-Tahoon W, El-Omda AE-A, et al. Thyroiditis as arisk factor in polycystic ovarian syndrome women. Al-Azhar Medical Journal. 2023;52:601-16.
- [12]Meier RK. Polycystic ovary syndrome. Nursing Clinics. 2018;53:407-20.
- [13]Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism. 2019;92:108-20.
- [14]Cowan S, Lim S, et al. Lifestyle management in polycystic ovary syndrome–beyond diet and physical activity. BMC Endocrine Disorders. 2023;23:14.
- [15]Manouchehri A, Abbaszadeh S, et al. Polycystic ovaries and herbal remedies: A systematic review. JBRA assisted reproduction. 2023;27:85.
- [16]Cincione I, Graziadio C, et al. Short-time effects of ketogenic diet or modestly hypocaloric mediterranean diet on overweight and obese women with polycystic ovary syndrome. Journal of Endocrinological Investigation. 2023;46:769-77.
- [17]Deng C, Xiang Y, et al. Altered peripheral b-lymphocyte subsets in type 1 diabetes and latent autoimmune diabetes in adults. Diabetes Care. 2016;39:434-40.
- [18]Frasca D, Diaz A, et al. Phenotypic and functional characterization of double negative b cells in the blood of individuals with obesity. Frontiers in Immunology. 2021;12.
- [19]Hu C, Pang B, et al. Immunophenotypic profiles in polycystic ovary syndrome. Mediators of Inflammation. 2020;2020:5894768.
- [20]Joham AE, Norman RJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10:668-80.
- [21]Li H, Guo Y, et al. Gnrh receptor-activating autoantibodies in polycystic ovary syndrome: Identification of functional epitopes and development of epitope mimetic inhibitors. Endocrine. 2022;75:959-63.
- [22]Lindheim L, Bashir M, et al. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (pcos): A pilot study. PLoS One. 2017;12:e0168390.
- [23]Risal S, Pei Y, et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25:1894-904.
- [24]Du D, Li X. The relationship between thyroiditis and polycystic ovary syndrome: A meta-analysis. Int J Clin Exp Med. 2013;6:880-9.
- [25]Arduc A, Aycicek Dogan B, et al. High prevalence of hashimoto's thyroiditis in patients with polycystic ovary syndrome: Does the imbalance between estradiol and progesterone play a role? Endocr Res. 2015;40:204-10.
- [26]Janssen OE, Mehlmauer N, et al. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. 2004;150:363-9.
- [27]Sarkar D. Recurrent pregnancy loss in patients with thyroid dysfunction. Indian journal of endocrinology and metabolism. 2012;16:S350-1.
- [28]Kim JJ, Yoon JW, et al. Thyroid autoimmunity markers in women with polycystic ovary syndrome and controls. Human Fertility. 2022;25:128-34.
- [29]Serin AN, Birge Ö, et al. Hashimoto’s thyroiditis worsens ovaries in polycystic ovary syndrome patients compared to anti-müllerian hormone levels. BMC Endocrine Disorders. 2021;21:1-8.
- [30]Abdolahian S, Tehrani FR, et al. Effect of lifestyle modifications on anthropometric, clinical, and biochemical parameters in adolescent girls with polycystic ovary syndrome: A systematic review and meta-analysis. BMC endocrine disorders. 2020;20:1-17.
- [31]Bertolaccini ML, Gomez S, et al. Antiphospholipid antibody tests: Spreading the net. Annals of the rheumatic diseases. 2005;64:1639-43.
- [32]Wu L, Fang X, et al. The ovarian immune pathology and reproductive failures. Immunology of recurrent pregnancy loss and implantation failure: Elsevier; 2022. p. 333-50.
- [33]Wadood SA, Kadhum NAK, et al. Immunoglobulins igg, iga, igm, complement c3 and c4 levels in sera of patients with polycystic ovary syndrome and the risk of cardiovascular diseases. Iraqi J Biotechnol. 2015;14:329-38.
- [34]Sun Y, Chen X, et al. Serum amyloid a is a novel inflammatory biomarker in polycystic ovary syndrome. Clin Lab. 2019;65.
- [35]Hestiantoro A, Kapnosa Hasani RD, et al. Body fat percentage is a better marker than body mass index for determining inflammation status in polycystic ovary syndrome. Int J Reprod Biomed. 2018;16:623-8.
- [36]Lejman-Larysz K, Pietrzyk D, et al. Influence of hscrp parameter on the occurrence of metabolic syndrome in patients with polycystic ovary syndrome. Biomedicines. 2023;11:1953.
- [37]Kem DC, Li H, et al. The role of gnrh receptor autoantibodies in polycystic ovary syndrome. J Endocr Soc. 2020;4:bvaa078.
- [38]Alugoju P, Krishna Swamy V, et al. Health benefits of astaxanthin against age-related diseases of multiple organs: A comprehensive review. Critical Reviews in Food Science and Nutrition. 2022:1-66.
- [39]Ascani A, Torstensson S, et al. The role of b cells in immune cell activation in polycystic ovary syndrome. Elife. 2023;12:e86454.
- [40]Przepiera-Bedzak H, Brzosko M. Antiphospholipid syndrome with antiβ2glicoprotein-1 antibodies as the cause for recurrent tibial vein thrombosis in sapho syndrome a case report. Acta Dermatovenerologica Croatica. 2016;24:305.