Phenotypic screening of extended-spectrum beta-lactamase producing Salmonella in retail shrimp
Abstract
The occurrence of extended-spectrum beta-lactamase (ESBL) producing bacteria is concerning the scientific community since it confers multiple drug resistance (MDR). The study investigated the prevalence of MDR-ESBL-producing Salmonella strains from shrimp in, Bangladesh. A total of 165 shrimp samples were processed from 55 shrimp specimens from different retail shops. The presence of Salmonella was confirmed by standard methods followed by antibiotic susceptibility testing. Isolates exhibiting resistance to third-generation cephalosporin were considered as Salmonella positive isolates which was later proven by the double disc synergy test. Out of the total of 39 isolates tested, 18 were found to be Salmonella positive and originated from 7 departmental stores. The remaining 21 positive isolates were obtained from the local market. The body had the highest rate of positive samples (30.91%), followed by the head (23.63%), and the tail (16.36%). Additionally, isolated Salmonella were resistant to rifampicin and cefixime but 100% susceptible to co-trimoxazole, ofloxacin, streptomycin, nalidixic acid, and chloramphenicol, while ciprofloxacin showed intermediate resistance. Among the drugs tested, vancomycin, cephalexin, ampicillin, and colistin exhibited extreme resistance. Finally, while 6 of the tested isolates demonstrated resistance against the recommended cephalosporin, three of them (7.69%) were Salmonella ESBL positive in the double disc synergy test. In conclusion, the rising incidence of MDR and the developing prevalence of ESBL-positive Salmonella may put a burden on the healthcare system by limiting access to effective antibacterial drugs.
INTRODUCTION
Drug resistance is currently one of the most pressing concerns, because of the emergence of multidrug-resistant (MDR) bacteria, capable of producing extended-spectrum beta-lactamase (ESBL). ESBL is an enzyme produced by a variety of Gram-negative bacteria, most often Enterobacteriaceae that confers enhanced resistance to numerous antibiotic classes [1]. These ESBL-producing organisms are worrisome since they survive against cephalosporin, which is frequently applied to alleviate acute salmonellosis [2]. Among the 'new' β-lactamases emerging in pathogenic Enterobacteriaceae, the molecular class ESBLs can hydrolyze third-generation penicillin, cephalosporin, and aztreonam though inhibited by clavulanic acid, cephamycins or carbapenems [3]. The presence of MDR ESBL-producing bacteria such as Salmonella, in shrimp can pose a significant obstacle, leading to a decline in their value and increased risk of disease transmission. As a consequence, with the restricted use of antibiotics to treat bacterial illnesses, the transmission of drug-resistant strains to humans will emerge as a serious threat to the effectiveness and affordability of treatments for human infections [4].
In Bangladesh, shrimp is referred to as "white gold" because of its considerable economic significance. However, it is essential to note that this shellfish may also serve as a potential reservoir for foodborne pathogens, including Salmonella [5]. Salmonella, a pathogenic bacterium belonging to the Enterobacteriaceae family, is naturally found in the gastrointestinal tract of animals and causes a variety of foodborne illnesses [6]. This pathogen is frequently prevalent in the gastrointestinal tracts of animals; therefore, these diseases are typically linked to eating contaminated food, especially raw or undercooked animal products. It may flourish in aquatic environments, and it has already been found in aquaculture [7]. According to estimates, this bacterium is responsible for 80,3 million incidents of foodborne illness, with the United States of America (USA) reporting 1.2 million cases, 23,000 hospitalizations, and 450 deaths per year [8]. Moreover, this industry has already been identified as the primary source of foodborne disease transmission [9]. Fish and shrimp can become contaminated with salmonella before, during, or after harvest as well as during post-harvest processing, packing, storing, and distribution [7, 10]. People are most commonly infected when handling and processing sick fish in the food sector under unsanitary conditions, preparing dishes, or orally by consuming infected fish or associated items [11]. Particularly, undercooked, or raw fish consumption, is the most common cause of fish-based food poisoning. Ingesting bacteria-infested fish is responsible for 12% of food-borne illnesses [12]. The surveillance of Salmonella is proposed to keep fish and shellfish safe in each step from primary production-processing to consumption [13]. In modern aquaculture, particularly in shrimp production, the use of antibiotics in feed and water is a prevalent approach to combat disease. Consequently, when bacteria are exposed to antibiotics, they acquire resistance [14]. Moreover, it is popularly believed that the sub-therapeutic doses with more extended exposure to antibiotics in farming practices have accelerated the spread of superbugs [15].
The continuous emergence of new resistant bacteria has the potential to negatively affect our capacity to deliver quality medical care. This is because infections with these strains of bacteria can result in an increased frequency of treatment failure and disease severity. Therefore, Bangladesh’s environment is heavily contaminated with microbes that are resistant to treatment. There is not enough information on the ESBL characteristics among Salmonella isolated from shrimp in the Sylhet region, Bangladesh. So, the main goal of this study was to assess prevalence and detect pathogenic Salmonella phenotypically in commercially available shrimp that are raised on farms and sold in Sylhet city's neighborhood stores.
MATERIALS AND METHODS
Ethics statement
In the context of animal handling, this study was performed in full compliance with the current regulations and legislation of Bangladesh (Cruelty to Animals Act 1920, Act No. I of 1920, Government of the People's Republic of Bangladesh). The approval of this study was granted by Ethics Committee of Sylhet Agricultural University, Bangladesh and the ethical approval number is AUP2019017.
Study area and study period
The shrimp (Penaeus monodon Fabricius) used in this study were collected from Sylhet city, Bangladesh. All the shrimp samples were taken from 11 retail stores (both departmental and local).
Collection and processing of sample
The sampling regime was carried out between December 2019 and March 2021. A total of 55 shrimp were collected from 11 retail shops (5 samples per shop) in Sylhet city. The samples were placed in sterile stomacher bags and sealed appropriately. All the samples were conveyed to the laboratory after collection in black polyethylene bags placed within ice packs [16]. Each individual shrimp was again dissected into 3 body parts (head, body, and tail) and each part was considered as a separate entity for the potential source of Salmonella contamination. Hereafter, 165 (55×3) samples were aseptically processed for the present study. In the laboratory, shrimp was divided into three body parts using sterilized scissors. A homogenous grinding of the separated body parts of the shrimp was made using mortar and pestle. Each sample from different body parts was blended aseptically in separate containers.
Isolation of Salmonella
A swab from each body part was collected into different test tubes to culture bacteria on nutrient broth and incubated for 24 hours for primary growth at 37°C. Bacteria from the primary broth culture were streaked on Salmonella-Shigella (SS) agar media, a day of incubation at 37°C, black centered, smooth, small round colonies were appeared. Continuous subculture on SS agar was carried out until a single, pure colony was found. The Salmonella spp. suspected colonies were further cultured on MacConkey agar [17].
Biochemical characterization of Salmonella isolates
The biochemical techniques employed for the genus-level identification of presumptive Salmonella colonies were performed by standard methods [18]. Biochemical tests performed in distinct presumptive Salmonella colonies that were picked from the Petri plates included gram staining, citrate utilization, urease test, indole production, methyl red test, voges-Proskauer test, oxidase test, motility test, catalase test and sulfide test.
Antibiotic susceptibility test
Each of the Salmonella isolates used for phenotypic tests was tested for multidrug resistance with the Kirby Bauer disc diffusion test as prescribed by the Clinical and Laboratory Standards Institute [19]. The inhibitory zone diameter around each of the Salmonella colonies was interpreted as sensitive, intermediate, or resistant based on zone diameter interpretive standards stipulated by the Clinical and Laboratory Standards Institute. Amikacin (30 mcg), ampicillin (25 mcg), cefalexin (30 mcg), cefixime (5 mcg), cefotaxime (30 mcg), ceftazidime (30 mcg), ceftriaxone (30 mcg), chloramphenicol (30 mcg), ciprofloxacin (5 mcg), colistin (10 mcg), co-trimoxazole (25 mcg), doxycycline (30 mcg), imipenem (10 mcg), nalidixic acid (30 mcg), nitrofurantoin (30 mcg), ofloxacin (5 mcg), rifampicin (5 mcg), streptomycin (10 mcg), tetracycline (30 mcg), and vancomycin (30 mcg) were the antibiotic discs that were tested. An isolate was considered as an MDR if it exhibited resistance to at least three groups of antibiotics, following the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (2021), and EUCAST (2021) [19, 20].
Phenotypic characterization of ESBL-producing Salmonella
ESBL phenotypes were initially detected by placing several antibiotics from the third-generation cephalosporin, namely cefixime (5 mcg), cefotaxime (30 mcg), ceftazidime (30 mcg), and ceftriaxone (30 mcg). One of the first-generation cephalosporin, cefalexin (30mcg), was also used for detecting ESBL phenotypes. Therefore, Salmonella isolates showing resistance or intermediate resistance to one or both of Cefotaxime/Ceftriaxone and Ceftazidime are considered as potential ESBL producing isolates. The Phenotypic characterization of ESBL-producing Salmonella isolates was determined by double disc synergy test (DDST) (CLSI, 2021) [19].
Statistical analysis
The total Salmonella positive samples, relative frequency of each antibiotic, number of MDR and ESBL positive isolates were calculated, and the prevalence was assessed. One-way ANOVA: Single Factor, and t-Test (Microsoft Excel, 2016) were used to detect variation in the prevalence of Salmonella positive samples among local and departmental shrimp sources.
RESULTS
Initial screening of Salmonella isolates from shrimp
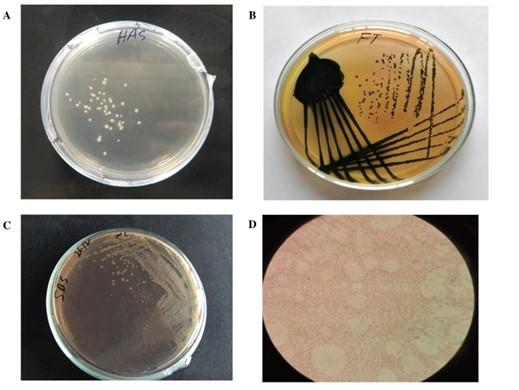
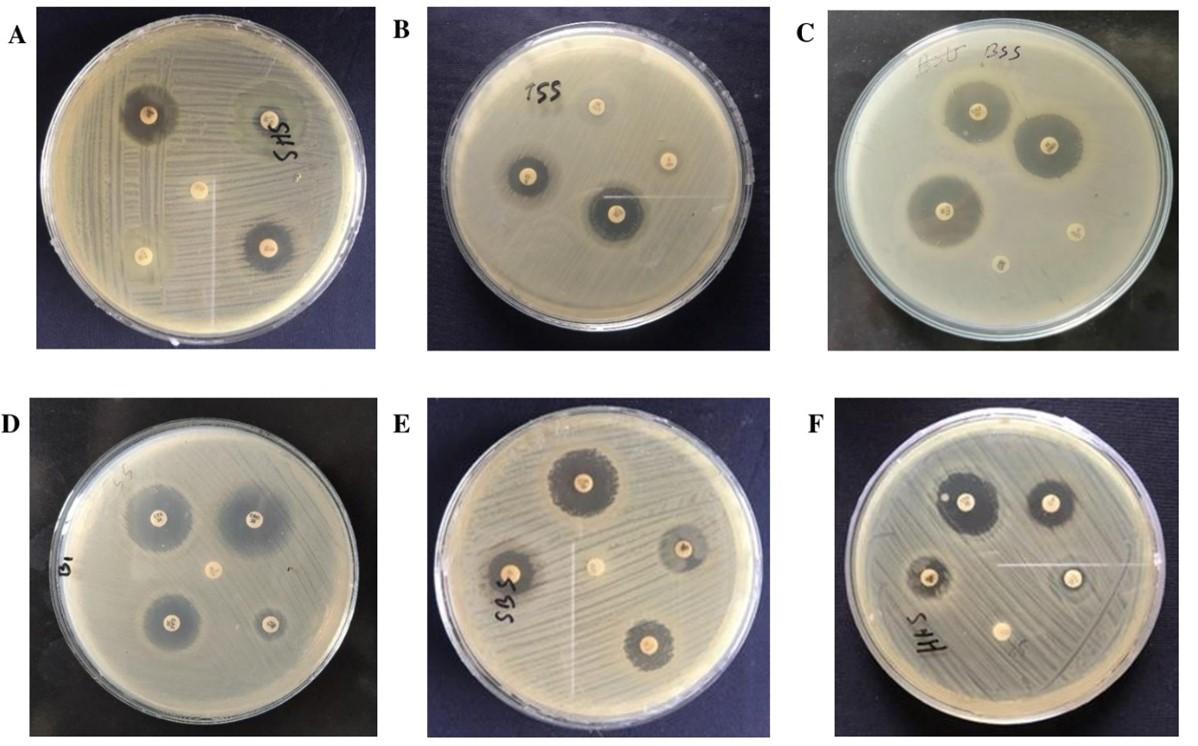
The unique morphology of Salmonella on nutrient agar such as circular in shape, low convex elevation, smooth surface, grayish white in color, and translucent structure was an initial indication for the presence of Salmonella (Table 1). Salmonella positive cases were indicated by the growth of smooth, black-centered colonies on SS agar media (Table 1, Figure 1). Salmonella was detected by the development of colorless colonies on MacConkey Agar (Figure 1, Table 1).
Table 1. Morphological characteristics of Salmonella on different culture media.
Biochemical characteristics of Salmonella isolates
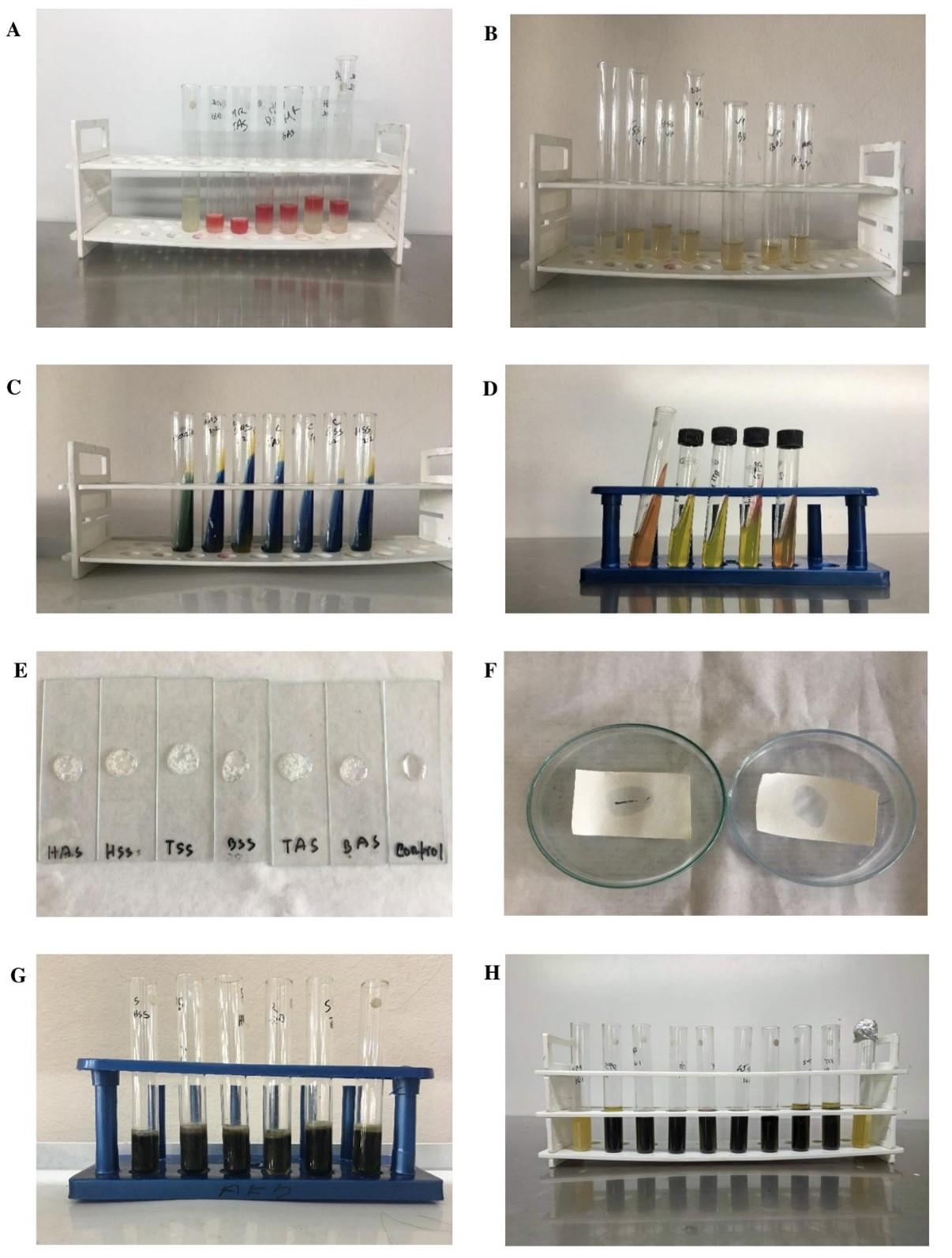
The biochemical tests were performed in distinct presumptive Salmonella colonies that were picked from the Petri plates including gram staining, citrate utilization, urease test, indole production, methyl red test, Voges-Proskauer test, oxidase test, motility test, catalase test and sulfide test. All test results confirmed Salmonella genus (Table 2, Figure 2).
Table 2. Biochemical characterization of Salmonella isolates using several methods.
Occurrence of Salmonella isolates in retail shops
Out of 165 tested samples, a total of 39 were found as Salmonella positive (prevalence 23.64%). Among them, out of the 105 samples examined from the 7 department stores, a total of 18 samples tested positive for Salmonella and the overall frequency was 17.14% in those departmental shops. A highest 5 Salmonella positive sample was isolated from Swapno, Uposohor branch while the samples collected from the Agora Kumarpara branch were free from Salmonella contamination. This is the only shop where there was no Salmonella positive sample comprising both local and departmental shops. On the other hand, sample collected from all the local shops were contaminated with Salmonella, where 21 out of 60 samples were found contaminated (35%). A maximum of ten isolates were identified from Bondor Bazar samples with the highest frequency of 66.67%. This is densely contaminated market among all the retail shops tested. The number of Salmonella positive isolates obtained from its corresponding source shop and their prevalence is summarized in Table 3.
Table 3. Prevalence of Salmonella in different retail shops of Sylhet city.
Prevalence of Salmonella in selected body parts of shrimp
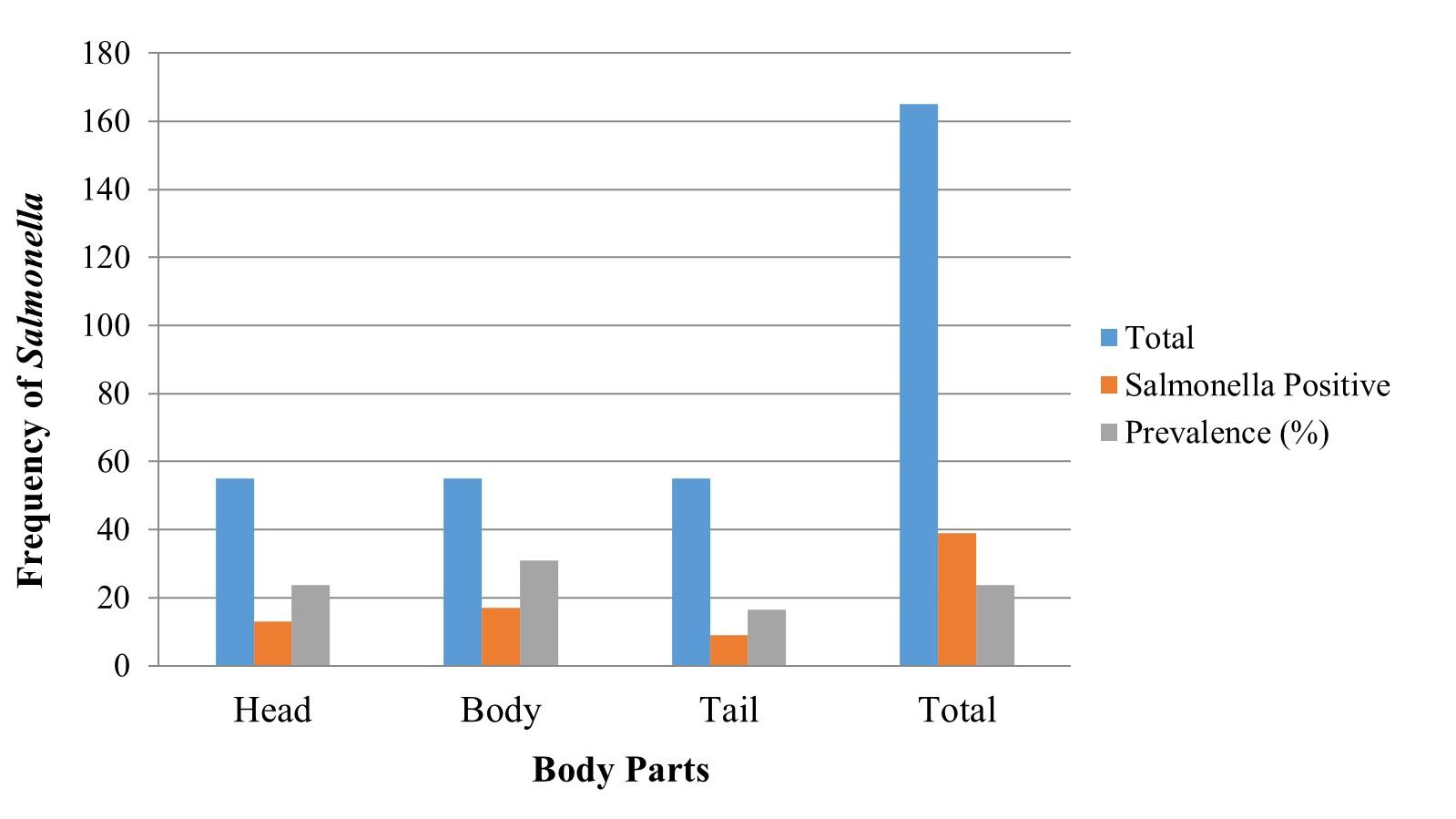
Among the thirty-nine positive isolates, thirteen of them were identified from body parts out of fifty-five samples while seventeen and nine isolates were obtained from head and tail, respectively. Among the three body parts of shrimp, the highest frequency of Salmonella was observed in the body (30.91%), followed by the head and tail 23.63%, and 16.36%, respectively (Figure 3).
Antibiotic susceptibility
It was found that all the Salmonella isolates were resistant against rifampicin, and cefixime although the isolates were found 100% susceptible to co-trimoxazole, ofloxacin, streptomycin, nalidixic acid, and chloramphenicol. All of them demonstrated an intermediate range of resistance to ciprofloxacin. Antibiotics that showed intermediate resistance were not considered as fully resistant in this study. Among other antibiotics, the majority of the Salmonella isolates were susceptible to amikacin (89.74%), nitrofurantoin (71.79%), imipenem (76.92%), doxycycline (66.67%), and cephalosporin such as ceftazidime, ceftriaxone (94.88% each), cefotaxime (84.62%) whereas vancomycin, cephalexin, ampicillin, and colistin were highly resistant as their frequency was observed as, 92.31%, 89.74%, 76.92%, and 61.53%, respectively (Figure 4).
Prevalence of multidrug-resistant Salmonella isolates
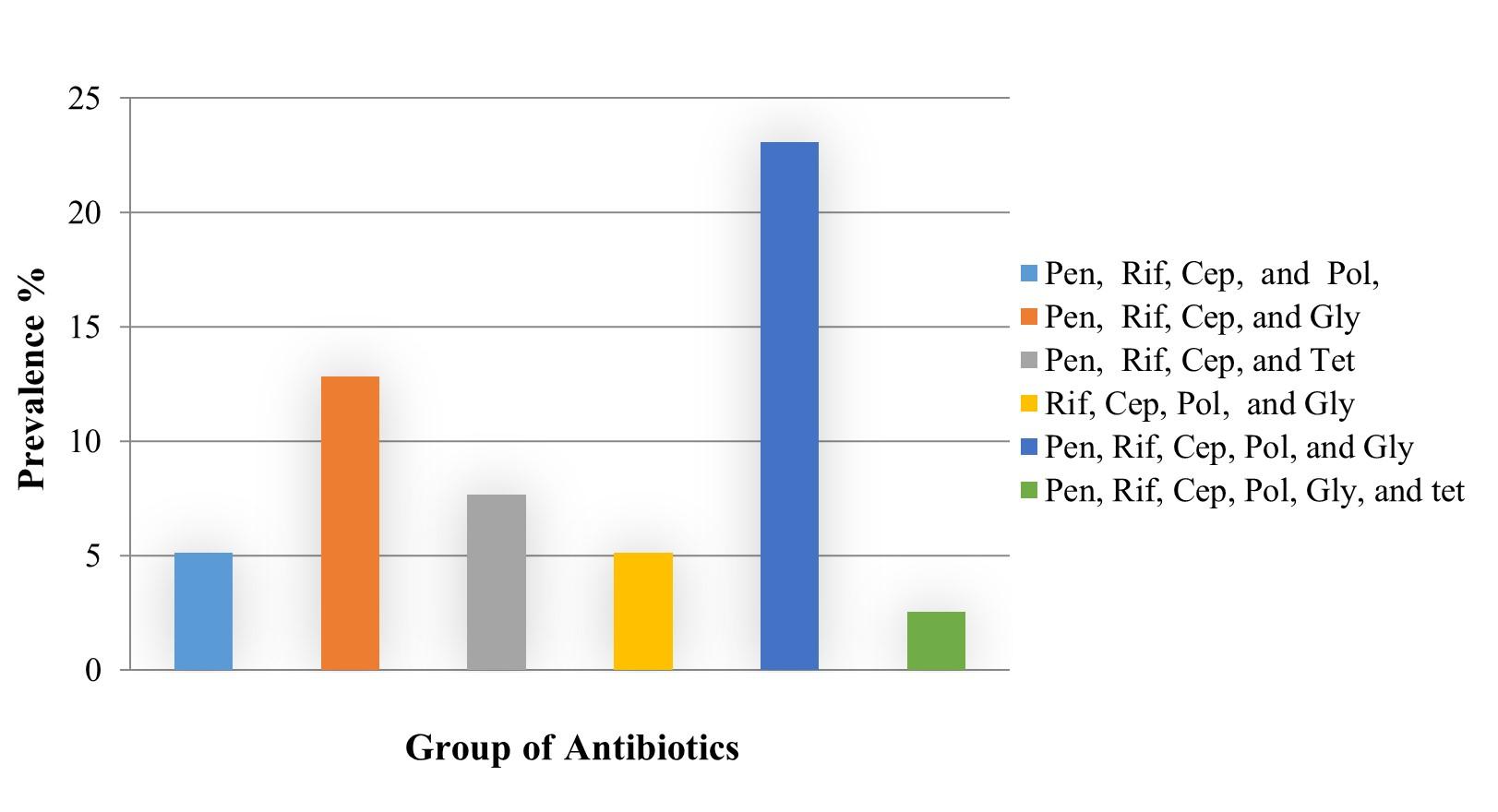
Among the 39 isolates according to the resistance frequency recorded against each drug. A total of 22 MDR Salmonella was identified the prevalence was determined as 56.41%. The MDR isolates were resistant against a minimum of four and up to six groups of antibiotics. The maximum number of MDR isolates (9) were prevalent in body parts of shrimp while the highest relative frequency of MDR was in the tail (77.77%). One isolate identified from the body part of a shrimp sample was resistant against 6 groups of antibiotics (penicillins, rifampicin, cephalosporin, polymyxins, glycopeptide, and tetracyclin) which was the highest among the MDR (Table 4, Figure 5).
Penicillins, rifampicin, cephalosporin, polymyxins, and glycopeptide were found as the most frequently resistant drug groups, resistant detected against a maximum of nine MDR isolates (3 from each body part) (Figure 6).
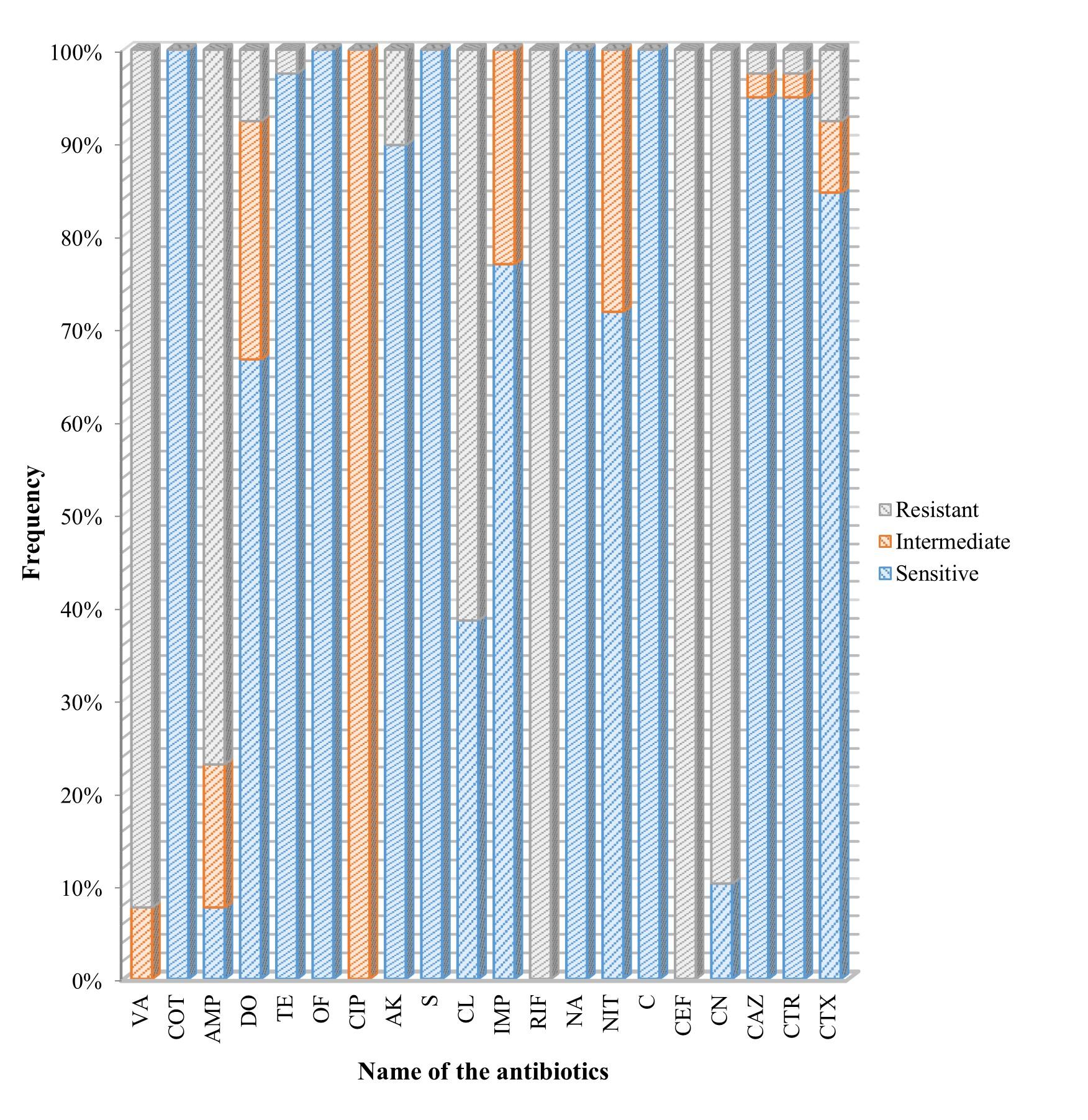
Table 4. Prevalence of MDR Salmonella and their distribution among body parts.
Phenotypic characterization of ESBL Salmonella
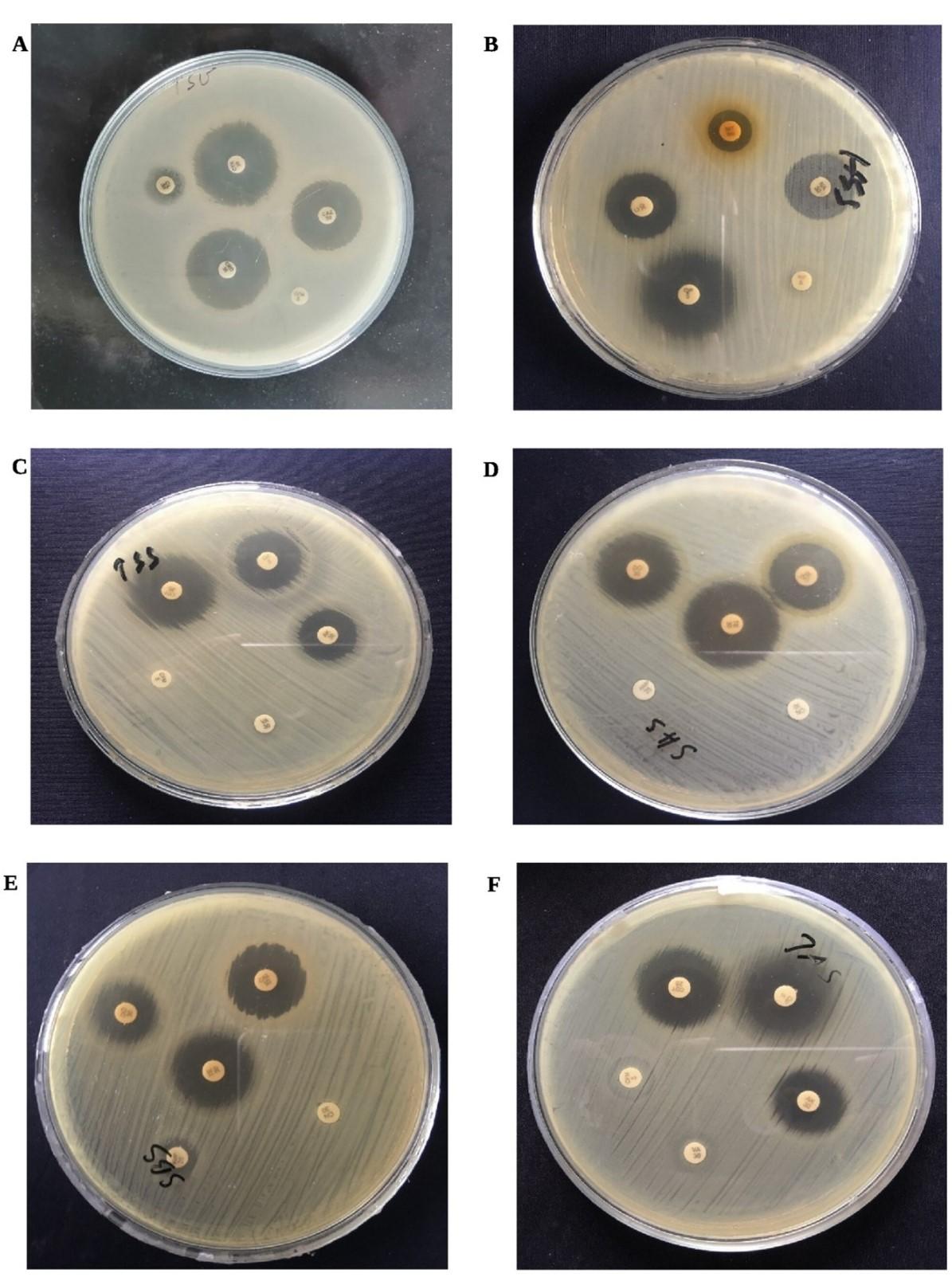
Only six of the tested isolates were considered to be ESBL producers as they demonstrated resistance against the recommended 3rd generation cephalosporin (Cefotaxime/Ceftriaxone and Ceftazidime) (Table 5, Figure 7).
Table 5. Phenotypic screening of ESBL suspecting isolates based on DDT method.
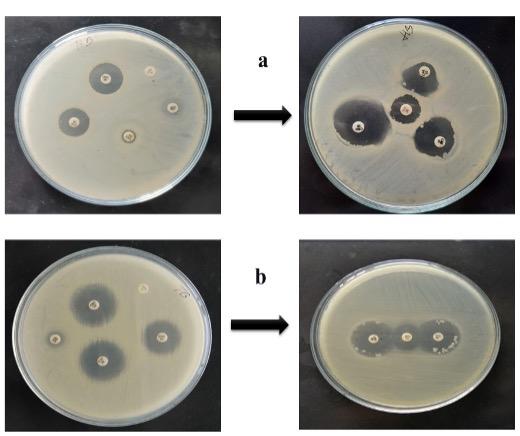
Interpreting the DDT method, 6 ESBL producing isolates were suspected. Among the six, three of them fulfilled the criteria for detection of ESBL producing Salmonella on DDST. These three isolates demonstrated a satisfactory level of expansion or distortion in the indicator cephalosporin’s inhibition zone towards AMC disc, indicating a synergy between the Augmentin disc and the indicator cephalosporin, hence phenotypically confirming their ESBL positivity status. So finally, 3 ESBL positive Salmonella was identified among the 39 isolates with a prevalence of 7.69% (Table 6). Both distortion and enhancement in the indicator cephalosporin’s inhibition zone is displayed in Figure 8.
Table 6. Prevalence of ESBL producing Salmonella.
DISCUSSION
Antibiotic resistance has been identified as among the three greatest medical threats of the twenty-first century [21]. Already the world has witnessed a rapid surge in morbidity and mortality caused by MDR bacterial infections. The prevalence of ESBL producing multidrug resistant enteric pathogens like Salmonella in shrimp is an emerging threat to public health.
However, there are lack of data regarding the prevalence of such MDR Salmonella in retail shrimp of Bangladesh. Our study showed that the frequency (23.63%) of Salmonella positive sample was moderate. Among the positive samples, in most of the cases, the pathogen was found in the body region (30.91%) of the shrimp, followed by head and tail portions with a frequency of 23.63% and 16.36% (Figure 3). The frequency might be varied because the body region is larger compared to both head and tail, hence there was a higher possibility of bacterial load while grinding the sample. The frequency was almost double in local shops than that of departmental shops. Eighteen samples were Salmonella positive from the 7 departmental shops with a frequency of 17.14% and 21 out of 60 samples were found contaminated in local shops at a frequency of 35%. This finding is indicating the poor hygienic condition in the local market. Earlier, Hossain et al. (2013) discovered that shrimp from Dhaka City, Bangladesh had a greater incidence of Salmonella (65.71%) which was higher than our findings from the Sylhet region [22]. When Pinu et al. (2007) studied with the microbiological state of frozen shrimp, they discovered a 55% frequency of Salmonella which was also higher than that of our findings [23]. The discrepancy in the frequency might be due to the sources of the sample. All the shrimp sample of the study by Hossain et al., 2013 was collected solely from 7 local markets of Dhaka city while in the present study sample were collected from a mixture of local and departmental shops (7 from departmental and 4 from local market) [22]. However, the data from previous studies suggested that the relative frequency of Salmonella in shrimp can significantly vary from one region to another. While the frequency was 14.58% in Nigeria, a comparatively increasing number of Salmonella was detected in white shrimp (40%), in Bangkok, and Thailand [24, 25].
While tested for the presence of antibiotic resistance, Hossain et al. (2012) found MDR Salmonella in black tiger shrimp cultured in Bangladesh with a frequency of 66.67% [26]. A study detected MDR in shrimp with a frequency of 64.3% for Salmonella enteritidis (tolerant to three groups), while the frequency was 27.3% for Salmonella typhimurium (7 different groups) [24]. Nguyen et al. (2016) found MDR Salmonella in shrimp at a frequency of 37.5%, which were resistant against a minimum of three to six groups of antibiotics [25]. Noor et al. (2014) reported an isolate from shrimp in Bangladesh, resistant to sixteen drugs implying that the crustacean’s can contribute as a vehicle for the dissemination of MDR strains [27]. Antibiogram analysis of our study revealed that Salmonella isolated from shrimp are still susceptible to several antibiotics as they were 100% sensitive to co-trimoxazole, ofloxacin, streptomycin, nalidixic acid, and chloramphenicol. In contrast, among the twenty antibiotics tested, all the isolates were resistant against cefixime and rifampicin. This data might be relieving but it is deceptive. In reality, drug resistance is an ongoing process and bacteria adopt resistance each time they are exposed to antimicrobial agents. Our experiment also validated this hypothesis as it was found that 89.74%, 76.92%, and 61.53% of isolates were resistant against vancomycin, cephalexin, ampicillin, and colistin, respectively. All the isolates showed intermediate resistance to ciprofloxacin, the drug commonly used for the treatment of human Salmonella infections. It is indicating that all these mentioned drugs are actually on their way to being failed. Among the 39 isolates, 22 MDR Salmonella (56.41%) was detected all of which were resistant against at least four groups of antibiotics. One isolate was resistant against a maximum of six antibiotic groups while a maximum of nine isolates was found resistant against five antibiotic groups. The emergence of MDR in shrimp might be the consequence of the sub-therapeutic doses with more extended exposure of antibiotics in farming practices heeding towards the rapid spread of drug-resistant strains.
In our study, three of the isolates acceptably fulfill the double-disc synergy test requirement, hence considered as ESBL positive (Table 6, Figure 8). It was found the prevalence of ESBL producing Salmonella in shrimp is 7.69%. Two of the ESBL producing isolates were found to be resistant against all three-indicator cephalosporin, cefotaxime, ceftazidime, and ceftriaxone, while one was found resistant against cefotaxime, and ceftazidime. However, in the presence of the Augmentin disc, AMC a distortion or enhancement in the zone of inhibition observed indicated that they are susceptible to clavulanic acid, major criteria to distinguish ESBL positive pathogen. Le et al. (2015) reported shrimp contamination with ESBL positive Escherichia coli (72.7%) detected in Vietnam which is markedly higher compared to the present study [28]. Besides, Rahman et al. (2020) characterize MDR E. coli isolated from chicken in Sylhet, Bangladesh. They reported MDR isolates at a frequency of 9.23%, while broiler chicken (12.8%) and layer chicken (7.61%) derived E. coli isolates carried genes that produce SHV type ESBL [29]. There is close proximity among several fish, poultry and beef markets in Sylhet city and there is a higher possibility of transmission of MDR pathogen by exchanging of host through horizon gene transfer. This study highlights the significant findings of current research, suggesting that ESBL producing pathogens may be emerging in the study area due to the similarities observed. The evolution of ESBL producing pathogens through this mechanism is plausible. The consequences of this situation are substantially deleterious and pose a major threat to the next generations. The emergence of drug resistance will soon pose a significant challenge to the availability of the susceptible drugs for testing salmonellosis.
CONCLUSION
Microbiological safety is an essential factor to consider, and shrimp as an aquaculture-derived product can act as a vehicle for the transmission of deadly pathogens. The outcomes of this study clearly indicate the presence of the pathogen in a considerable number of samples, and the strains isolated from those samples exhibit full or partial resistance to several commonly used antibiotics. Salmonella is among the 4 leading causes of diarrheal illness globally, and salmonellosis can be fatal as well. The emergence of drug-resistant is another pressing issue, and Salmonella is such a pathogen that has produced resistance already. The improved use of antibiotics may contribute to the emergence of more MDR pathogens. Consequently, the rise of drug resistance may pose a significant challenge to the availability of effective drugs for testing salmonellosis in the near future. This is the high time to ensure the responsible and legitimate use of antibiotics in the agriculture and aquaculture sectors to prevent the emergence of drug resistance. As a result of the study, valuable recommendations for the use of antibiotics in food animals were made. Based on these guidelines, the selective use of antibiotics as therapeutics in food animals may lower the cost of treatment, enable to prevent the development of ESBL-producing bacteria in food animals, and help to ensure the safety of human food. Further studies might be needed to apply the outcomes of the current study.
ACKNOWLEDGEMENT
This research was financially supported by University Grant Commission (UGC), Bangladesh (Project ID: SAURES-ID-343) under the control of Sylhet Agricultural University Research system (SAURES), Bangladesh.
AUTHOR CONTRIBUTIONS
DA and MMR designed outlines and drafted the manuscript. DA and MA performed the experiments and analyzed the data. AB, NIB, and MSS wrote the initial draft of the manuscript, SM, KMAZ, MMHK and SS reviewed the scientific contents described in the manuscript. All authors read and approved the final submitted version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Giamarellou H. Multidrug resistance in Gram‐negative bacteria that produce extended‐spectrum β‐lactamases (ESBLs). Clin. Microbiol. Infect. 2005; 11: 1-16.
- [2]Paterson DL. Resistance in gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control. 2006; 34(5): 20-28
- [3]Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001; 14(4): 933–951.
- [4]Mathew AG, Cissell R, et al. Antibiotic resistance in bacteria associated with food animals: A United States perspective of livestock production. Foodborne Pathog. Dis. 2007; 4(2): 115-133.
- [5]Amagliani G, Brandi G, et al. Incidence and role of Salmonella in seafood safety. Food Res. Int. 2012); 45(2): 780-788.
- [6]Pelzer KD. Salmonellosis. J. Am. Vet. Med. Assoc. 1989; 195(4): 456-463.
- [7]Mol S, Cosansu S, et al. Survival of Salmonella Enteritidis during salting and drying of horse mackerel (Trachurus trachurus) fillets. Int. J. Food Microbiol. 2010; 139(2): 36-40.
- [8]Scallan E, Hoekstra RM, et al. Foodborne illness acquired in the United States major pathogens. Emerg Infect Dis. 2011, 17(1): 7-15.
- [9]Seel SK, Kabir SML, et al. Molecular detection and characterization of Salmonella spp. isolated from fresh fishes sold in selected upazila markets of Bangladesh. Bangladesh Journal of Veterinary Medicine 2016; 14(2): 283-287.
- [10]Norhana MW, Poole SE, et al. Prevalence, persistence and control of Salmonella and Listeria in shrimp and shrimp products: A review. Food Control. 2010; 21(4): 343-361.
- [11]Fernandes DVGS, Castro VS, et al. Salmonella spp. in the fish production chain: a review. Cienc. Rural. 2018; 48(8): 7-12.
- [12]Aberoumand A. Estimation of microbiological variations in minced lean fish products. World J. Fish Mar. Sci. 2010; 2(3): 204-207.
- [13]Popović NT, Skukan AB, et al. Microbiological quality of marketed fresh and frozen seafood caught off the Adriatic coast of Croatia. Vet. Med. 2010; 55(5): 233-241.
- [14]Allen HK, Donato J, et al. Call of the wild: antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010; 8(4): 251-259
- [15]Barbosa TM, Levy SB. The impact of antibiotic use on resistance development and persistence. Drug Resist. 2000; 3(5): 303-311.
- [16]WHO (World Health Organization) (2010). Laboratory protocol: Isolation of Salmonella spp. From food and animal faeces, 5th Ed. 4-8, 13. Accessed on 27 April 2017. http://www.antimicrobialresistance. dk/data/images/protocols/isolation_of_salm_2206 0.pdf.
- [17]Islam MM, Islam MN, et al. Isolation and identification of Escherichia coli and Salmonella from poultry litter and feed. Int. J. Nat. Soc Sci. 2014; 1(1): 1-7.
- [18]Krieg NR and Holt JC. Bergey’s Manual of Systematic Bacteriology, 1st Ed., Vol. 1, Williams and Wilkins, Baltimore. United States, 1984. pp 150-180.
- [19]Clinical and Laboratory Standards Institute (CLSI) (2021) Performance standards for antimicrobial susceptibility testing, CLSI standard M100. Clinical and Laboratory Standards Institute, Wayne, PA. pp 39.
- [20]European Committee on Antimicrobial Susceptibility Testing (EUCAST) (2021) Breakpoint tables for interpretation of MICs and zone diameters, version 11.0.
- [21]Lachmayr KL, Kerkhof LJ, et al. Quantifying nonspecific TEM β-lactamase (bla TEM) genes in a wastewater stream. Appl. Environ. Microbiol. 2009; 75(1): 203-211.
- [22]Hossain MS, Khaleque HM, et al. Prevalence of Multidrug Resistant Salmonella in Shrimp of Dhaka City. Microbiology Journal. 2013; 3(1): 21-28.
- [23]Pinu FR, Yeasmin S, et al. Microbiological conditions of frozen shrimp in different food market of Dhaka city. Food Sci. Technol. Res. 2007; 13(4): 362-365.
- [24]Beshiru A, Igbinosa IH, et al. Prevalence of antimicrobial resistance and virulence gene elements of Salmonella serovars from ready-to-eat (RTE) shrimps. Front. Microbiol. 2019; 10: 1613.
- [25]Atwill ER, Jeamsripong S. Bacterial diversity and potential risk factors associated with Salmonella contamination of seafood products sold in retail markets in Bangkok, Thailand. Peer. 2021; 9: e12694.
- [26]Hossain MS, Aktaruzzaman M, et al. Prevalence of multiple drug resistant pathogenic bacteria in cultured black tiger shrimp (Penaeus monodon Fabricius). Global Journal of Environmental Research. 2012; 6(3): 118-124.
- [27]Noor R, Hasan MF, et al. Molecular characterization of the virulent microorganisms along with their drug-resistance traits associated with the export quality frozen shrimps in Bangladesh. SpringerPlus. 2014; 3(1): 469.
- [28]Le QP, Ueda S, Nguyen TNH, et al. Characteristics of extended-spectrum β-lactamase–producing Escherichia coli in retail meats and shrimp at a local market in Vietnam. Foodborne Pathog. Dis. 2015; 12(8): 719-725.
- [29]Rahman MM, Husna A, et al. Isolation and molecular characterization of multidrug-resistant Escherichia coli from chicken meat. Sci. Rep. 2020; 10(1): 1-11.