Human mesenchymal stem cell secretome lowers caspase-3 levels and apoptosis in hepatocytes of cholestatic rats
Abstract
Liver cirrhosis is a major cause of morbidity and mortality in the world. Definitive therapy is liver transplantation, but it is constrained by difficulties in finding a transplant donor. Human mesenchymal stem cell-based therapy can help liver regeneration directly, through hepatogenic differentiation, or indirectly through the paracrine secretome. Thus, this study aims to determine the effect of Human mesenchymal stem cell secretome (HuMSC-S) administration on caspase 3 levels and apoptotic hepatic cells in a rat model with cholestasis after choledochal duct ligation receiving urso deoxy cholic acid (UDCA). Twenty-four male Wistar rats were subjected to a choledochal duct ligation and were included in this randomized experimental study. After surgical intervention, all rats were randomly assigned into 4 group: control, UDCA, HuMSC-S, and a combination of UDCA and HuMSC-S for 4 weeks. Caspase-3 levels were assessed from the blood sample, while the apoptosis of hepatocytes was evaluated using histopathologic examination of the liver. Interestingly, caspase-3 level was significantly lower in the UDCA and HuMSC-S-treated groups compared to the UDCA or HuMSC-S alone. Similarly, the apoptosis of hepatocyte was significantly lower in the combination group compared to the UDCA or HuMSC-S alone. In conclusion, addition of HuMSC-S to UDCA lowered caspase-3 levels and apoptotic cell count in rats with hepatic cholestasis after choledochal duct ligation.
INTRODUCTION
Cholestasis is a clinical syndrome caused by reduced bile secretion from liver cells, impaired bile secretion at the cholangiocyte level, obstruction of bile flow by stones (cholelithiasis), or tumor masses. When bile formation or excretion occurs, accumulation of biliary constituents will exceed liver's normal cellular architecture, contributing to liver parenchyma cellular damage. Cirrhosis is the final stage of chronic and progressive liver disease caused by infection, autoimmune disorders, biliary obstruction, and metabolic disorders. Globally, cirrhosis is ranked 15th as a cause of morbidity and 11th as a leading cause of mortality [1]. Definitive therapy for cirrhosis is liver transplantation, but it takes time to find a matched donor, and thus, patients have to wait relatively for a long time and should continue to use urso deoxy cholic acid (UDCA) as an effort to slow the disease progression [2, 3]. However, studies have found that UDCA can inhibit DNA repair mediated by poly (ADP-ribose) polymerase, thereby interfering with the effectiveness of regeneration of the damaged cells. Therefore, alternative medical therapy that can stimulate liver regeneration is needed.
Human mesenchymal stem cells (HuMSC) have low immunogenicity, self-renewal, and are easy to obtain, making it a promising therapy for liver regeneration [4]. HuMSC- based therapy can help liver regeneration directly, through hepatogenic differentiation, or indirectly through the paracrine secretome [5]. Secretome is a soluble molecule and exosome produced by HuMSC, which can support hepatic epithelial regeneration through hepatogenic differentiation. The secretome was also found to inhibit cell apoptosis [6], suggesting that adipose-derived stem cell secretome (ADSC-S) can reduce liver cell apoptosis within 1-3 days after surgery.
The process of hepatic fibrogenesis is affected by the presence of oxidative stress that increases proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), interferon, and interleukin (IL). Previous studies in rats showed that increased inflammation, oxidative stress, and liver fibrosis in diabetic rats could be diminished by ramipril treatment. Another found that HMG-CoA reductase inhibitor (rotuvastatin) treatment could be used as an alternative therapy to decrease oxidative stress parameters and liver enzyme activities [7-10]. These cytokines induce caspase-3 activation as an effector caspase that activates hepatic stellate cells (HSCs) and cause fibrogenesis [11]. Suppression of caspase-3 is known to have protective and anti-apoptotic effects on liver cells and reduces collagen deposition, ultimately leads to inhibition of fibrogenesis [12]. Because of this, we aimed to study the effect of human mesenchymal stem cell secretome (HuMSC-S) administration on caspase 3 levels and apoptosis of hepatocytes in a rat model with cholestasis after choledochal duct ligation receiving standard UDCA therapy.
MATERIALS AND METHODS
Randomized experimental research with a post-test only control group design was carried out from April to December 2022 at Experimental Animal Laboratory, Gajah Mada University, Indonesia.
Preparation of UDCA and HuMSC-S
The UDCA (Dexa Medica, Tangerang, Indonesia) dose for this study was based on the range of doses used in human subjects that were converted to experimental animals, resulting in a dose of 4.5 mg in rats weighing 200 grams [13].
Preparation of HuMSC-S was carried out in the laboratory of Gadjah Mada University, Yogyakarta, and was processed through 4 phases and cultured, in which HuMSC-S were derived from human umbilical cord that reached 60% harvested using the warm trypsinization. After trypsin neutralization, the cell suspension was centrifuged at 3000 rpm for 10 minutes. The supernatant was removed, and the cell precipitate was washed with PBS three times. The cell precipitate was then resuspended in a new medium with a concentration of 10,000 cells per ml. Stem cells are modified into embryoid bodies and grown in culture media with complete media until a junction between the embryoid bodies was formed. Mesenchymal stem cell-conditioned media (MSC-CM) production was carried out by washing the embryoid body culture with sterile PBS and filling the embryoid body culture plate with 10 mL of complete medium without serum. After 48h, MSC-CM was stored at -20 °C until use [14]. The dose of HuMSC-S was based on the effective dose range in regeneration in other studies, namely 0.2 ml/kg [15].
Animal models and study procedures
Twenty-four male Wistar rats aged two months and weighing 200-250 grams were used as experimental models and were then adapted, given food and drink ad libitum for 5 days. The Wistar rats were managed in accordance with animal welfare regulation [16]. Serum samples from the tail blood were obtained to measure the ALT and AST levels before intervention using kinetic methods for transaminase assay. For the surgical procedure, an injection of ketamine hydrochloride (0.5 cc) intramuscular (IM) was given for anesthesia and cefotaxime (18 mg) intravenous (IV) for prophylactic antibiotic. After disinfection of the surgical area, a midline incision was made to reach the peritoneum and liver. After the common bile duct was visualized, it was then separated from the portal vein and hepatic artery using micro-serrated forceps with a 0.5 mm, curved tip. A 4-0 Silk suture was placed around the bile duct and tied with two surgical knots for maximum obstruction. Suturing was done to close the incision wound. After two weeks, all 24 rats were randomly divided into four groups (6 rats per group): Control (K1), UDCA therapy (K2), HuMSC-S therapy (K3), and combination of UDCA and HuMSC-S (K4). UDCA (4.5 mg, orally) and HuMSC-S (0.2 ml/kg intraperitoneal) were given 1 dose per week for 4 weeks. At the end of the treatment Wistar rats were killed with cervical dislocation under general anesthesia.
The experiments were conducted following the institutional guidelines and the study has been approved by Health Research Ethical Committee, Faculty of Medicine, Universitas Diponegoro (Protocol Number: 09/EC/H/FK-UNDIP/1/2023). The Wistar rats were managed in accord with animal welfare regulations.
Measurement of serum ALT and AST
Serum alanine transaminase (ALT) and aspartate aminotransferase (AST) levels were analysised using kinetic methods with DiaSys Kit (Diagnostic Systems GmbH, Alte Strasse 9, 65558 Holzheim, Germany) to measure the liver damage.
Evaluation of caspase 3 levels
At the end of the treatment, blood samples were taken through the medial canthus sinus orbitalis using a syringe. A blood sample was inserted into a tube, then was centrifugated for 10 minutes at a speed of 3000 rpm at a temperature of 40 C, and then the serum was taken. Assessment of caspase-3 levels was examined using Direct ELISA technique with ELISA Kit (FineTest© from Wuhan City, Hubei province, China). Optical density absorbance was measured using a Microplate Reader at 450 nm.
Measurement of apoptosis of hepatocytes
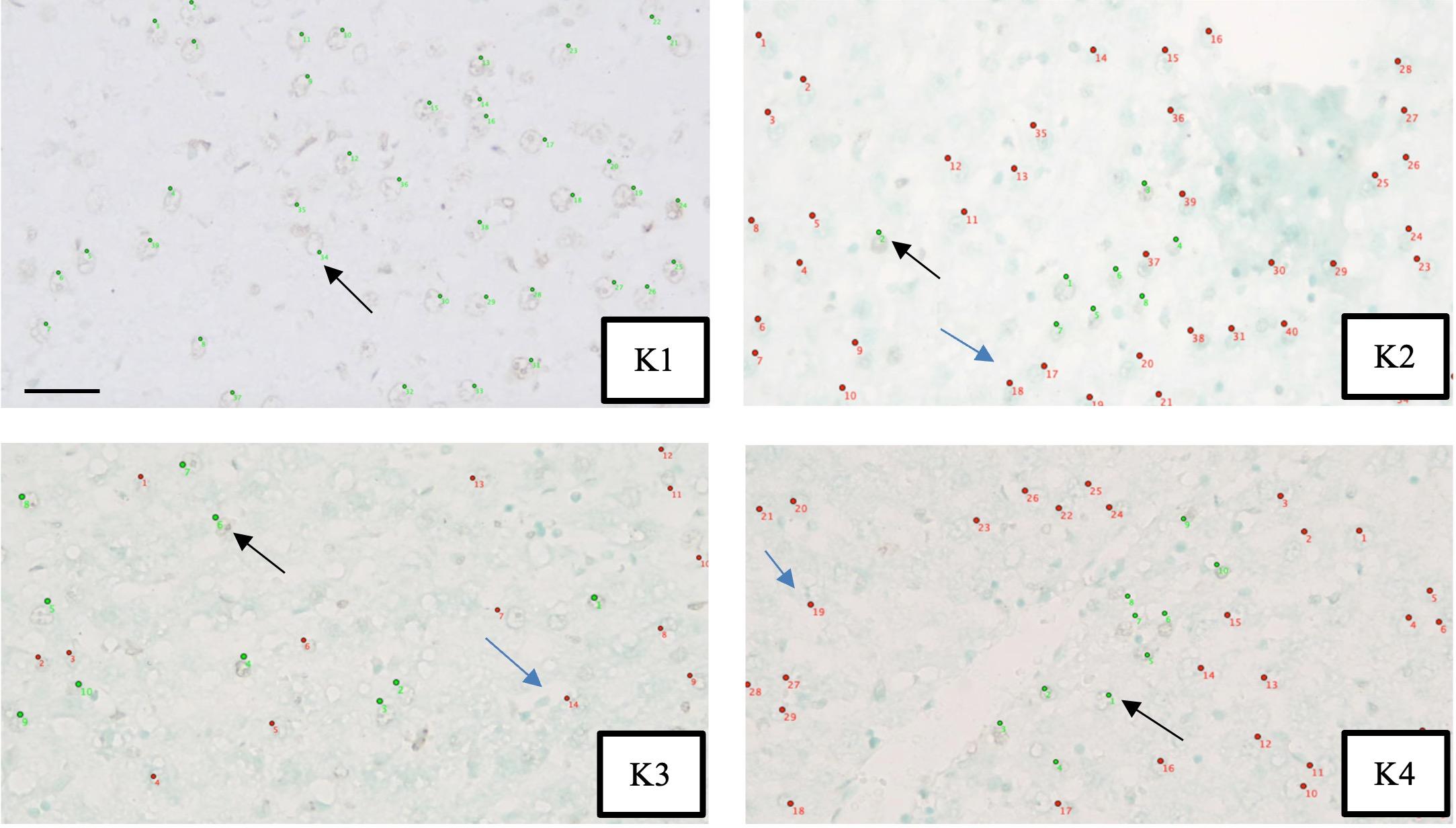
Liver biopsies were done after secretome injection to determine histopathological features using the Terminal deoxynucleotidyl Transferase-mediated dUTP Nick End Labeling (TUNEL) staining. The amount of apoptotic hepatocyte was assessed through observation using a microscope at 400x magnification. An apoptotic hepatocyte was marked by green fluorescence color using TUNEL staining BioVision Apo-BrdU-IHC Kit from Milpitas Blvd., Milpitas, CA 95035 USA. The assessment was carried out by two anatomical pathologists independently, using the hotspot method on 10 visual fields by counting the cells stained with fluorescence color. The results were then analyzed for inter-observer agreement.
Statistical analysis
The data distribution of caspase 3 levels and apoptotic hepatic cell count were analyzed using the Shapiro Wilk test. For normally distributed data, the parametric statistical analysis was performed. Otherwise, the Kruskal Wallis test followed by the Mann Whitney test was used to determine differences between groups. The difference is considered significant if the p value <0.05 with a 95% confidence interval. The suitability test for apoptosis data uses the Inter-Class Correlation Coefficient test. If the kappa value is > 0.90, it can be concluded that it is almost perfect.
RESULTS
Baseline characteristics
The body weight of all rats was measured on the 6th day of acclimatization, which was also the first day of the treatment process. The analysis result showed a normal and homogeneous distribution of the body weight of the rats (Table 1). Table 1 showed that male gender and rats body weight data were normally and homogeneously distributed (p> 0.05).
ALT and AST levels were measured before and after ligation to assess liver damage. Table 2 showed significant differences in ALT levels in all groups, with data distributed normally and homogeneously (p> 0.05). From Table 2, the results showed that there was an increase in ALT and AST levels after ligation of the common bile duct, which indicates liver damage.
Table 1. Baseline body weight.
Table 2. ALT and AST levels at pre-and post-ligation of the bile duct in rats.
Effect of HuMSC-S on caspase-3 levels
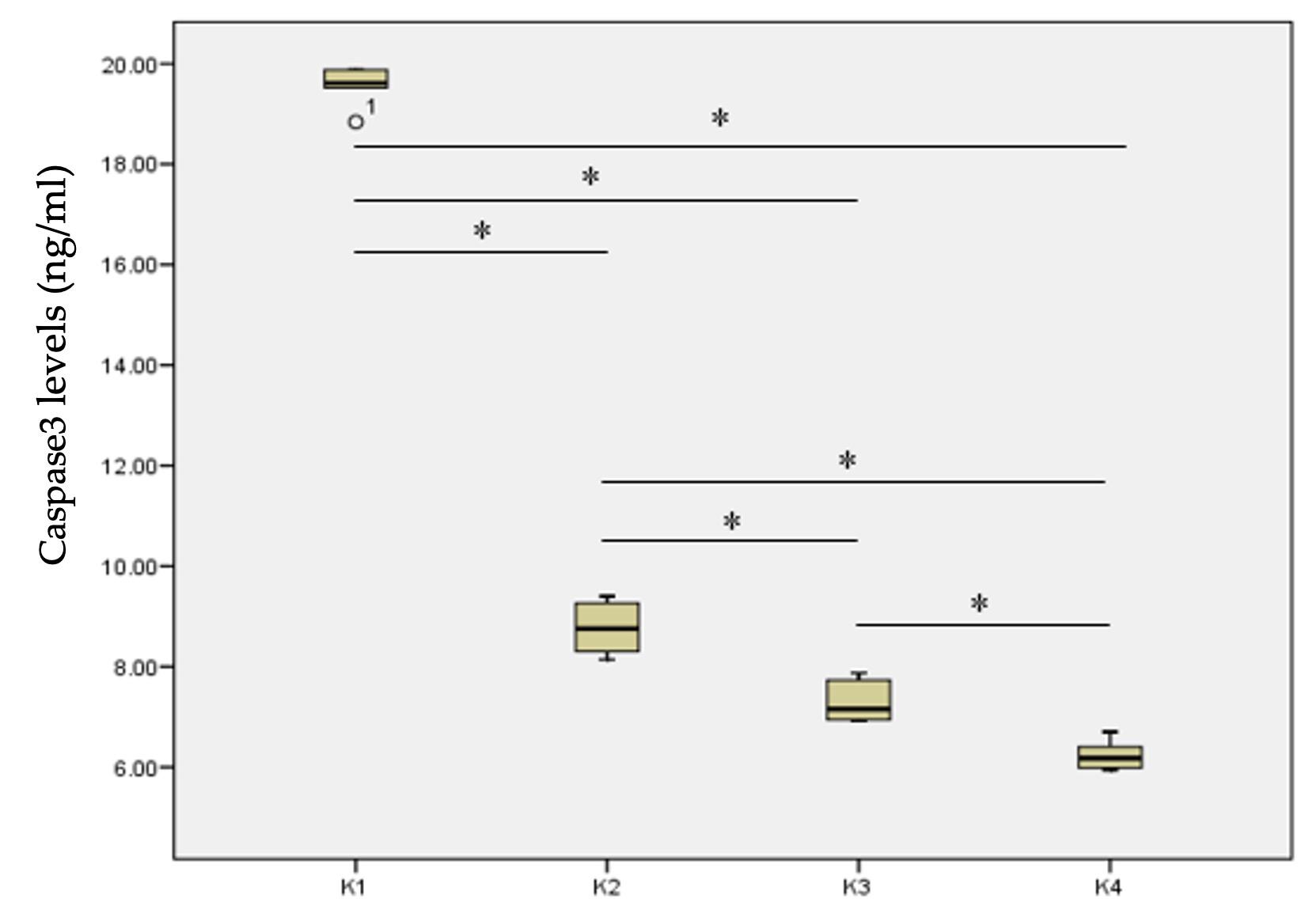
The data on caspase-3 levels was normally distributed and there was a significant difference in the caspase-3 levels between the study groups. The lowest caspase 3 level was found in the K4 group (6.23 ± 0.30 ng/ml), while the highest was in the K1 group (19.56 ± 0.38 ng/ml). Post Hoc analysis showed a significant difference in caspase 3 levels in the K1 group compared to the K2, K3, and K4 groups (Figure 1). Also, there was a significant difference in the caspase-3 levels in the K2 group compared to the K3 and K4 groups, and in the K3 group compared to the K4 group (Figure 1). HuMSC-S treated group (K3) had lower caspase 3 expression indicating that therapeutic effect of HuMSC-S was greater than UDCA. Finally, combination of UDCA and HuMSC-S (K4)-treated group (K4) had lower caspase-3 expression than K3 group.
Effect of HuMSC-S on apoptosis of hepatocytes
Assessment of apoptosis of hepatocytes was carried out by 2 pathologists independently, with an average score of observer-I was 27.85 ± 17.45 and an average score of observer-II was 28.24 ± 16.45. The intraclass correlation coefficient test obtained a value of κ = 0.990, indicating a very good agreement between the two observers. Apoptosis of hepatocyte was assessed using histopathologic examination involving TUNEL staining and hotspot method (Figure 2).
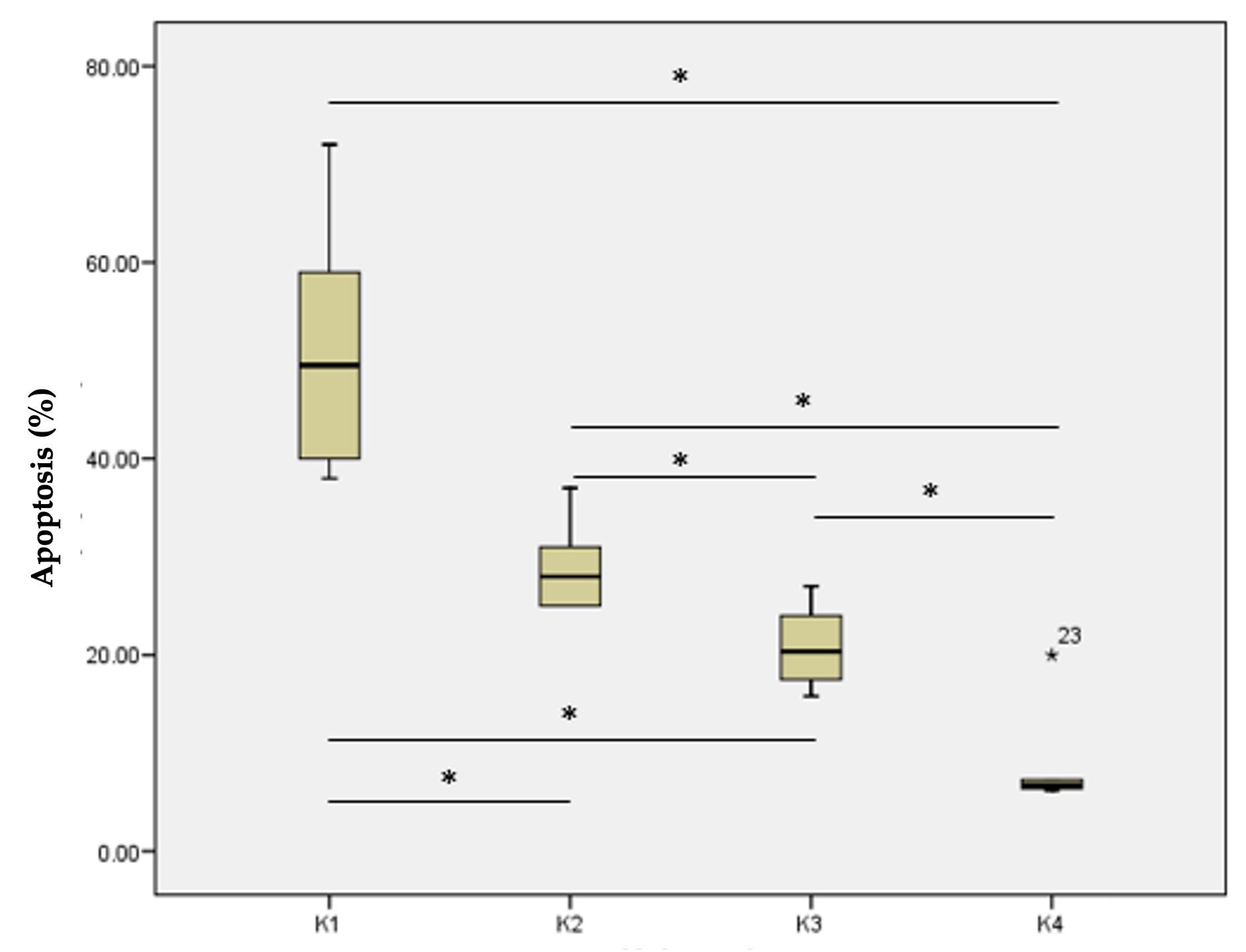
The data on apoptosis of hepatocytes was not normally distributed, hence the difference on apoptosis of hepatocytes between each group was analyzed using Kruskal Wallis test. There was a significant difference in apoptotic hepatocyte count between the study groups. Interestingly, K1, K2, and K3 groups had significantly higher numbers of apoptotic cells compared to group K4. The highest number of apoptotic cells was in the K1 group (51.33± 12.96%), while the lowest was found in the K4 group (8.88 ± 5.46%). It showed that the combination of UDCA and HuMSC-S was able to produce a decrease in apoptosis (Figure 3).
The Mann Whitney test showed a significant difference in apoptosis of hepatocyte in the K1 group to the K2, K3, and K4 groups. There was a significant difference in apoptosis of hepatocyte in the K2 group to the K3 and K4 groups, and in the K3 group to the K4 group (Figure 3).
Correlation of caspase-3 levels and apoptosis of hepatocytes
Analysis of caspase-3 levels from all groups found an average of 10.46 ± 5.46 ng/ml and an average of hepatic cell apoptosis of 27.51 ± 17.38% (Table 3). The normality test using the Shapiro-Wilk test showed that caspase-3 levels data were not normally distributed, and liver cell apoptosis data were normally distributed. Thus, to test the relationship of caspase-3 levels to liver cell apoptosis was carried out with the Pearson correlation test. The results of the Pearson correlation test obtained p value <0.001 with r value = 0.865. So, it can be concluded that there was a significant relationship between caspase-3 levels and liver cell apoptosis. This significant relationship showed that caspase-3 levels have important effects on the occurring of apoptosis process.
Table 3. Descriptive and normality test of caspase-3 and hepatic cell apoptosis.
DISCUSSION
This study aimed to examine the effect of HuMSC-S administration on caspase 3 levels and apoptotic hepatic cell count in a rat model with cholestasis after choledochal duct ligation receiving standard UDCA therapy.
This study found that groups K2, K3, and K4 had significantly lower caspase-3 levels than group K1. Caspase-3 levels were found to be lowest in the K4 group, indicating that the combination of UDCA and HuMSC-S produced the most significant decrease in caspase-3 levels. Several studies have found that one of the effects of mesenchymal stem cells (MSCs) administration is to inhibit the apoptosis of hepatocyte cells and promote their proliferation. Jiang et al. showed that administration of HuMSC-S resulted in decreased caspase-3 and increased Bcl-2 levels [17], indicating that HuMSC-S inhibited apoptosis of hepatocytes. In the line with this result, another study showed using western blot and immunohistochemical analysis that MSCs administration resulted in lower levels of the pro-apoptotic marker caspase-3 and pro-inflammatory markers [18]. These findings can be explained by the ability of cells to protect themselves when exposed to an irritant or toxin. This property can be expressed by the release of the secretome in response to an external stimulus. Because mesenchymal stem cell secretome has more robust responsiveness and plasticity to external stimuli than mature cells, in general, and these will produce a greater therapeutic potential effect.
This study used apoptosis of hepatocytes to assess the degree of cellular destruction. The presence of pro-apoptotic stressors, such as in liver cirrhosis will increase mitochondrial permeability and release of various apoptotic factors, which trigger activation of caspase effectors like caspase 3. Activation of caspase 3 has a major role in the process of cell damage and fibrosis of the liver, which will trigger a cutting process of a number of substrates in the cell, causing changes in the morphological characteristics of apoptosis. Suppression of caspase 3 levels has been shown to produce protective effects against hepatocellular damage, cell apoptosis, as well as pro-inflammatory signals [19].
We found that the lowest apoptosis of hepatocyte was in the K4 group, while the other groups had significantly higher count. These results indicated that the combination of UDCA and HuMSC-S reduced liver cell apoptosis. UDCA is known to play a role in cholestasis therapy by stimulating hepatic cell secretion, HCO3-secretion, anti-apoptotic effects, and reducing the toxicity of bile to reduce patient symptoms [20]. Administration of HuMSC-S reduced the level of apoptosis that occurred in patients with type 1 diabetes mellitus [21]. HuMSC-S is known to play a role in liver regeneration through modulation of the immune system and inflammation, anti- apoptotic activity, pro-angiogenic, antioxidants, and induced cell proliferation. Under conditions with a strong inflammatory response, including tissue damage, caspase signal activation occurs to induce apoptosis. MSCs can modulate apoptotic signals through the secretion of paracrine mediators, such as growth factors, but also through direct contact with affected cell types, such as T cells and B cells. In its anti-apoptotic role, MSCs, through IL-6 mediated mechanisms which will induce STAT-3 translocation and reduce ROS [22-24].
In this study, it was found that there was a significant relationship between caspase 3 levels and liver cell apoptosis. These results are in accordance with theory and other research which states that caspases are a family of cysteine proteins, functions in programmed cell death. Caspase works in coordinating cellular structures destruction such as DNA fragmentation and degradation of cytoskeletal proteins. This role makes caspase 3 correlates in apoptosis process. Higher caspase 3 levels have been found in many patients with various liver diseases such as steatohepatitis, acute liver failure, or chronic alcoholic hepatitis. In a study of these patients, a correlation was found between activated caspase 3 and the degree of liver apoptosis as an indicator of worse prognosis [25, 26].
CONCLUSION
From the research, it was found that administrating HuMSC-S lowered caspase 3 levels and apoptosis of hepatocytes in rats with hepatic cholestasis after choledochal duct ligation that received standard UDCA therapy. Caspase 3 levels also had a significant correlation with hepatic cell apoptosis in male Wistar rats with hepatic cholestasis. Adding HuMSC-S administration could be considered as a therapy in patients who received standard UDCA therapy, however, further research should determine the effective therapeutic dose for liver cell apoptosis.
ACKNOWLEDGEMENTS
Thanks to the Department of Biological Sciences Gadjah Mada University for providing the facility needed to conduct the research. Thanks to Magfirah, PhD and Hanggoro T Rinonce, PhD from the Anatomical Pathology Department, Gadjah Mada University for making and analyzing the histologic samples.
AUTHOR CONTRIBUTIONS
All the authors designed outlines and drafted the manuscript, performed the experiments, analyzed the data, wrote the initial draft of the manuscript, and reviewed the scientific contents described in the manuscript. All authors read and approved the final submitted version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Cheemerla S, Balakrishnan M. Global epidemiology of chronic liver disease. Clinical liver disease. 2021;17:365-70.
- [2]D'Amico G, Morabito A, et al. Clinical states of cirrhosis and competing risks. Journal of hepatology. 2018;68:563-76.
- [3]Kotb MA. Molecular mechanisms of ursodeoxycholic acid toxicity & side effects: Ursodeoxycholic acid freezes regeneration & induces hibernation mode. International journal of molecular sciences. 2012;13:8882-914.
- [4]Driscoll J, Patel T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. Journal of gastroenterology. 2019;54:763-73.
- [5]Yao X, Wang J, et al. The anti-fibrotic effect of human fetal skin-derived stem cell secretome on the liver fibrosis. Stem cell research & therapy. 2020;11:379.
- [6]Jiao Z, Ma Y, et al. Protective effect of adipose-derived mesenchymal stem cell secretome against hepatocyte apoptosis induced by liver ischemia-reperfusion with partial hepatectomy injury. Stem cells international. 2021;2021:9969372.
- [7]Shojaie L, Iorga A, et al. Cell death in liver diseases: A review. International journal of molecular sciences. 2020;21.
- [8]Chakraborty JB, Oakley F, et al. Mechanisms and biomarkers of apoptosis in liver disease and fibrosis. International journal of hepatology. 2012;2012:648915.
- [9]Siddiqua S, Sikder B, et al. Ramipril, an angiotensin-converting enzyme inhibitor, ameliorates oxi dative stress, inflammation, and hepatic fibrosis in alloxan-induced d iabetic rats. J Adv Biotechnol Exp Ther.5:510.
- [10]Sikder B, Akter F, et al. Hmg-coa reductase inhibitor, rosuvastatin averted carbon tetrachloride -induced oxidative stress, inflammation and fibrosis in the liver of r ats. J Adv Biotechnol Exp Ther.3:01.
- [11]Thapaliya S, Wree A, et al. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced nash model. Digestive diseases and sciences. 2014;59:1197-206.
- [12]Woolbright BL, Ding WX, et al. Caspase inhibitors for the treatment of liver disease: Friend or foe? Expert review of gastroenterology & hepatology. 2017;11:397-9.
- [13]Prihatno SA, Padeta I, et al. Effects of secretome on cisplatin-induced testicular dysfunction in rats. Veterinary world. 2018;11:1349-56.
- [14]Zhang S, Yang Y, et al. The clinical application of mesenchymal stem cells in liver disease: The current situation and potential future. Annals of translational medicine. 2020;8:565.
- [15]Kim D, Cho GS, et al. Current understanding of stem cell and secretome therapies in liver diseases. Tissue engineering and regenerative medicine. 2017;14:653-65.
- [16]Service USDoAAaPHI. Usda animal care: Animal welfare act and animal welfare regulations “blue book.”. 2019.
- [17]Jiang W, Tan Y, et al. Human umbilical cord msc-derived exosomes suppress the development of ccl(4)-induced liver injury through antioxidant effect. Stem cells international. 2018;2018:6079642.
- [18]Kim HJ, Kim OH, et al. Harnessing adipose‑derived stem cells to release specialized secretome for the treatment of hepatitis b. International journal of molecular medicine. 2021;47.
- [19]Barreyro FJ, Holod S, et al. The pan-caspase inhibitor emricasan (idn-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver international : official journal of the International Association for the Study of the Liver. 2015;35:953-66.
- [20]Bellentani S. Immunomodulating and anti-apoptotic action of ursodeoxycholic acid: Where are we and where should we go? Eur J Gastroenterol Hepatol. 2005;17:137-40.
- [21]Al-Azzawi B, McGuigan DH, et al. The secretome of mesenchymal stem cells prevents islet beta cell apoptosis via an il-10-dependent mechanism. The Open Stem Cell Journal. 2020;6:1-12.
- [22]Han Y, Yang J, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal transduction and targeted therapy. 2022;7:92.
- [23]Lin L, Du L. The role of secreted factors in stem cells-mediated immune regulation. Cellular immunology. 2018;326:24-32.
- [24]González-González A, García-Sánchez D, et al. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World journal of stem cells. 2020;12:1529-52.
- [25]Shi Y. Caspase activation, inhibition, and reactivation: A mechanistic view. Protein science : a publication of the Protein Society. 2004;13:1979-87.
- [26]Lorente L, Rodriguez ST, et al. High serum caspase-3 levels in hepatocellular carcinoma prior to liver transplantation and high mortality risk during the first year after liver transplantation. Expert review of molecular diagnostics. 2019;19:635-40.